NRF2 in COST Action 20121
Our quarterly newsletter attempts to provide our latest news and also aims at becoming a forum for analysis of relevant topics on the field of NRF2 and provide comments to some of the most relevant articles published during the quarter. Previous newsletters can be accessed at:
https://benbedphar.org/our-first-newsletter/
https://benbedphar.org/issue-2-abril-2022/
https://benbedphar.org/issue-3-july-2022/
https://benbedphar.org/issue-4-october-2022/
https://benbedphar.org/issue-5-january-2023/
https://benbedphar.org/issue-6-april-2023/
https://benbedphar.org/issue-7-october-2023/
https://benbedphar.org/issue-8-january-2024/
https://benbedphar.org/issue-9-april-2024/
https://benbedphar.org/issue-10-october-2024/
https://benbedphar.org/issue-11-january-2025/
Antonio Cuadrado
Chair of COST Action 20121, BenBedPhar
Autonomous University of Madrid
Comments from the Working Groups
Media Composition and Oxygen Tension Modulate Nrf2 Activation in HepG2ARE Cells.
Cell culture models offer greater experimental flexibility than animal models and, thus, are indispensable in preclinical research and drug discovery. However, the translational value of cell models is often not optimal due to differences in intracellular processes in cell culture and tissue. To improve the biochemical fidelity of cell models, cell media with metabolite composition that mimics blood plasma composition was developed – Plasmax by Ximbio (UK) and HPLM by Gibco[1, 2]. Cancer cells grown in such media show metabolomics profiles similar to cancer biopsy in contrast to cells grown in regular media (DMEM). In addition to media, the oxygen tension in regular cell culture is much higher than in tissues, around 19% (19kPa) in an incubator versus 3-7% (3- 7kPa) in most tissues[3]. This higher oxygen tension also modifies cell metabolism, including cellular stress response.
In this study, we evaluated cell viability, stress response, and Nrf2 activation in response to various stressors in hepatic HepG2ARE cells that were grown in DMEM or Plasmax media at ambient air (19% oxygen) or physioxia (5% oxygen)[4].
Glutathione and malondialdehyde levels were elevated in cells grown in Plasmax media, suggesting their increased sensitivity to oxidative stress. The activation of Nrf2 by various stressors also depended on the cell media and oxygen tension. Not without exceptions, in general, the cultivation of cells in Plasmax media had a more pronounced effect on cell stress response than the cultivation of cells at reduced oxygen levels. Thus, we suggest that adopting Plasmax (or HPLM) media is the first easy step in increasing the metabolic fidelity of cell culture models. Maintaining lower oxygen tension (physioxia) is also highly desirable; however, it requires considerable investment in equipment.
References:
1. Vande Voorde, J., et al., Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci Adv, 2019. 5(1): p. eaau7314.
2. Cantor, J.R., et al., Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase. Cell, 2017. 169(2): p. 258-272 e17.
3. Ast, T. and V.K. Mootha, Oxygen and mammalian cell culture: are we repeating the experiment of Dr. Ox? Nat Metab, 2019. 1(9): p. 858-860.4. Taba, R., et al., Effect of Media Composition and Oxygen Tension on Cellular Stress Response and Nrf2 Activation in HepG2ARE Cells. Antioxidants (Basel), 2025. 14(2).
Anton Terasmaa
Laboratory of Chemical Biology,
National Institute of Chemical Physics and Biophysics,
Tallinn, Estonia
WG1, on behalf of authors
A molecular glue for the enhanced β-TrCP-mediated NRF2 degradation
NRF2 is controlled at the protein stability level by several E3 ubiquitin ligases, including CRL3Keap1, SCFβ-TrCP, and HRD1 (1,2) (Figure 1). Under homeostatic conditions CRL3Keap1 constitutively targets NRF2 for proteasomal degradation. However, the high prevalence of loss-of-function mutations in KEAP1, or gain-of-function mutations in NRF2, and the corresponding increased transcriptional activity of NRF2 in human lung cancer (3,4), conferring a broad-spectrum resistance to therapy, suggests that boosting the activity of some of the other ubiquitin ligases could be a viable strategy for reducing tumour growth.
A high-throughput transcriptomics and viability screen of more than 125,000 compounds has led to the discovery and optimization of ARP-4922, a molecular glue that promotes the β-TrCP-mediated proteasomal degradation of NRF2 (5). This compound forms a ternary complex with NRF2 and β-TrCP, resulting in the accelerated degradation of NRF2. ARP-4922 is orally bioavailable and reduces tumour growth in mouse xenograft models of human lung adenocarcinoma and squamous cell carcinoma with hyperactive NRF2. This discovery, which was reported at the American Association for Cancer Research Annual Meeting held in April 25-30, 2025 at the McCormick Place Convention Center in Chicago, Illinois, USA, was made at Arpeggio Biosciences who are progressing ARP-4922 and related compounds into late-stage preclinical development.
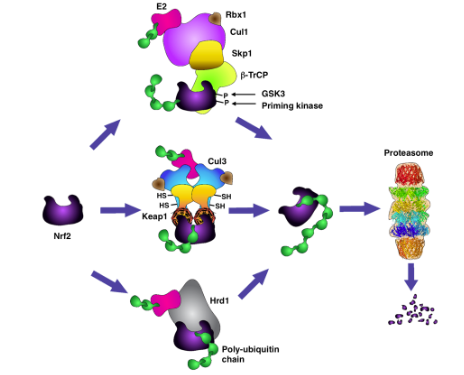
Figure 1. Under homeostatic conditions, Nrf2 is targeted for ubiquitination and proteasomal degradation by sevarl ubiquitin ligase systems, including CRL3Keap1, SCFβ-TrCP, and HRD1. Degradation of Nrf2 by Keap1 occurs when the cysteine sensors of Keap1 are in the reduced state. Degradation of Nrf2 by β-TrCP requires the formation of a phosphodegron on Nrf2 following phosphorylation catalyzed by GSK3, and a priming kinase. Degradation of Nrf2 by Hrd1 occurs during ER stress. ARP-4922 acts as a molecular glue, bridging NRF2 and β-TrCP, and resulting in the accelerated ubiquitination and proteasomal degradation of NRF2. Figure from Reference 2.
References
- Hayes et al. Regulating Nrf2 activity: ubiquitin ligases and signaling molecules in redox homeostasis. Trends Biochem Sci 2025; 50: 179-205.
- Holmstrom et al. The multifaceted role of Nrf2 in mitochondrial function. Curr Opin Toxicol 2016; 1, 80-91.
- Cancer Genome Atlas Research. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012; 489, 519-525.
- Cancer Genome Atlas Research. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014; 511, 543-550.
- Read et al. Discovery and optimization of ARP-4922: an orally bioavailable molecular glue degrader of NRF2. 2025 AACR Annual Meeting, Chicago Illinois, USA
Albena T Dinkova-Kostova
University of Dundee, United Kingdom
WG2 Leaders, on behalf of authors
Thirty years of NRF2: advances and therapeutic challenges
The influential journal Nature Reviews Drug Discovery, with an impressive impact factor of 122.7, recently published a comprehensive review by Donna D. Zhang on the mechanisms of Nrf2 signaling compounds and their implications for human health.
The review emphasises the dual role of NRF2 in cancer, highlighting that while it acts as a protective factor in normal cells, it also promotes survival and therapeutic resistance in certain cancers, particularly those with KEAP1 or NRF2 mutations. Companies such as Reata Pharmaceuticals and Kyowa Kirin have explored NRF2 modulators (e.g. bardoxolone methyl), but have had mixed results in clinical trials. The need for specific NRF2 inhibitors for resistant cancers creates opportunities for new therapies and biotechnological developments.
The review also lists approved NRF2 inducers such as dimethyl fumarate (DMF) and diroximel fumarate, which
are used to treat multiple sclerosis, and omaveloxolone, which is approved for the treatment of Friedreich’s ataxia. These drugs already generate revenue (e.g. Biogen’s Tecfidera). Sulforaphane is currently in clinical trials for diabetes and autism and, if approved, could expand its market, benefiting both nutraceutical and pharmaceutical companies.
The review also emphasises the importance of ferroptosis and iron metabolism, and how NRF2 regulates key pathways in ferroptosis, such as glutathione synthesis and iron homeostasis, which are relevant in cancer and neurodegenerative diseases. Companies developing ferroptosis inhibitors (e.g. in combination with NRF2 inhibitors) could attract investment, particularly in oncology. The review also highlights innovative approaches to new NRF2-related therapies. Strategies such as protein-fragment complementation assays (PROTACs) and molecular glues to degrade NRF2 or KEAP1 are mentioned, although these remain in the preclinical stage. Companies such as Arvinas, a leader in PROTACs, could explore this area to attract venture capital and pharmaceutical collaborations.
This publication once again highlights the importance of advances in NRF2 modulation, presenting these compounds as potential game-changers in the therapeutic market. In particular, it emphasizes their relevance for cancer and chronic diseases, with opportunities ranging from approved drugs to emerging technologies such as targeted protein degradation.
Ioannis Trougakos
The National and Kapodistrian
University of Athens, GR
Santiago Cuevas
BioMedical Research Institute of Murcia , ES
WG4 Co-leaders. Economic Exploitation
NRF2: A Key Player in Healthy Aging and Disease Prevention
Aging is a complex, unavoidable biological process marked by the progressive accumulation of damage to cellular components, including DNA, RNA, proteins, and lipids. This damage disrupts key metabolic pathways, leading to increased frailty and a higher risk of death. Aging is also the greatest risk factor for developing chronic diseases such as neurodegenerative and cardiovascular disorders.
In recent years, attention has shifted from simply extending lifespan to preserving healthspan — the period of life spent in good health — free from serious disease or disability. Among the key hallmarks of aging, we focused our attention on the role played by the loss of proteostasis associated with the failure of stress and redox responses. In our recently published review (1) we highlight how the interplay between the transcription factor NRF2 (nuclear factor erythroid 2-related factor 2) and proteostasis is emerging as a crucial axis in the fight against age-related diseases.
Proteostasis, or protein homeostasis, refers to the ability of the cells to maintain a functional and balanced protein environment. This is achieved through the coordinated action of molecular chaperones, which assist in protein folding, and quality control systems like the unfolded protein response, which help prevent the buildup of damaged or misfolded proteins. When refolding mechanisms fail, damaged proteins are targeted for degradation via the ubiquitin-proteasome system and/or autophagy. These mechanisms are especially critical in the brain, where protein aggregates and oxidative stress contribute to neurodegeneration in diseases like Alzheimer’s and Parkinson’s.
In youthful, healthy cells, NRF2 orchestrates a broad protective program, regulating antioxidant responses, detoxification, mitochondrial function, and protein quality control. But with aging, NRF2 activity declines. This compromises the cell’s ability to respond to oxidative stress, inflammation, and protein misfolding, accelerating the development of age-related conditions such as atherosclerosis and neurodegeneration.
To counteract these effects, researchers are exploring therapies aimed at re-activating NRF2 or boosting proteostasis (Figure 1). Natural compounds like sulforaphane can stimulate NRF2 activity, while other strategies include chaperone inducers, autophagy enhancers, and even gene therapy or CRISPR-based approaches targeting the NRF2 pathway.
However, NRF2 must be modulated with precision. While its activation can restore cellular defenses, excessive NRF2 activity may pose risks, such as supporting cancer cell survival. Similarly, overactivation of proteostasis mechanisms may interfere with essential cellular functions. Therefore, therapeutic interventions must strike a careful balance between efficacy and safety.
Despite encouraging preclinical results, several challenges remain in bringing NRF2-targeted therapies to the clinic. These include optimizing drug delivery, minimizing side effects, and fully understanding the long-term implications of NRF2 activation.
Still, the NRF2-proteostasis axis represents one of the most favourable avenues in the field of aging research. Fine-tuning these protective systems, holds great promises to delay cellular aging, prevent disease onset, and enhance resilience in aging populations paving the way for a new era of targeted therapies for age-related diseases.
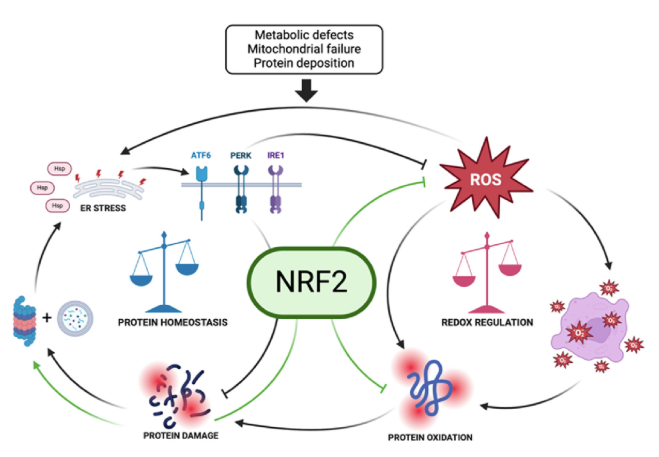
Figure 2. Illustration summarizing the key functions of NRF2 in maintaining protein homeostasis and redox equilibrium. Green arrows indicate NRF2-mediated regulatory actions within cellular homeostatic pathways (image created using BioRender, Toronto, ON, Canada).
References
- Buttari B, Tramutola A, Rojo AI, Chondrogianni N, Saha S, Berry A, Giona L, Miranda JP, Profumo E, Davinelli S, Daiber A, Cuadrado A, Di Domenico F. Proteostasis Decline and Redox Imbalance in Age-Related Diseases: The Therapeutic Potential of NRF2. Biomolecules. 2025 Jan 13;15(1):113. doi: 10.3390/biom15010113. PMID: 39858508; PMCID: PMC11764413.
Brigitta Buttari
Istituto Superiore di Sanità, IT
WG5 Leader, on behalf of authors
Hot from Pubmed
A redox-independent stress response mediated by phase-separated SQSTM1/p62
The KEAP1 (kelch like ECH associated protein 1)- NFE2L2/NRF2 (NFE2 like bZIP transcription factor 2) pathway is a major antioxidative stress pathway that contributes to cellular homeostasis. KEAP1 acts as a sensor and attenuates degradation of the transcription factor NRF2, which induces gene expression for a network of enzymes involved in the antioxidant response. When cells are exposed to various electrophiles and reactive oxidative species, they modify one or more selective cysteine residues in KEAP1, resulting in conformational changes that disable its NRF2-inhibitory function. In addition to this redox-dependent pathway, SQSTM1/p62 (sequestosome 1), which is a selective autophagy receptor for ubiquitinated proteins and a driver of liquid-liquid phase separation (LLPS) upon binding to ubiquitinated proteins, competitively inhibits the binding between KEAP1 and NRF2, thereby disabling the NRF2-repressive function of KEAP1. Our study showed that phase-separated SQSTM1/p62 bodies are phosphorylated by ULK1 (Unc-51 like autophagy activating kinase 1) and that KEAP1 is retained in the SQSTM1/p62 body, resulting in NRF2-activation in a redox-independent manner.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/40395541/
Oxygen needs sulfur, sulfur needs oxygen: a relationship of interdependence
Oxygen and sulfur, both members of the chalcogen group (group 16 elements), play fundamental roles in life. Ancient organisms primarily utilized sulfur for energy metabolism, while the rise in atmospheric oxygen facilitated the evolution of aerobic organisms, enabling highly efficient energy production. Nevertheless, all modern organisms, both aerobes and anaerobes, must protect themselves from oxygen toxicity. Interestingly, aerobes still rely on sulfur for survival. This dependence has been illuminated by the recent discovery of supersulfides, a novel class of biomolecules, made possible through advancements in technology and analytical methods. These breakthroughs are reshaping our understanding of biological processes and emphasizing the intricate interplay between oxygen and sulfur in regulating essential redox reactions. This review summarizes the latest insights into the biological roles of sulfur and oxygen, their interdependence in key processes, and their contributions to adaptive responses to environmental stressors. By exploring these interactions, we aim to provide a comprehensive perspective on how these elements drive survival strategies across diverse life forms, highlighting their indispensable roles in both human health and the sustenance of life.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/40394395/
Ferroptosis in acute liver Failure: Unraveling the hepcidin-ferroportin axis and therapeutic interventions
Acute liver failure (ALF) represents a critical clinical syndrome marked by massive hepatocyte death and severe functional deterioration. While metabolic dysregulation is a recognized hallmark, the pathophysiological implications of iron metabolism disturbance in ALF progression remain poorly understood, which may unveil novel therapeutic targets. Using clinical samples and preclinical murine models, we identified ferroptosis as a predominant pathological feature in ALF-affected livers. Notably, pharmacological inhibition of ferroptosis significantly attenuated disease progression in experimental ALF. Mechanistically, dysregulation of the hepcidin-ferroportin (FPN) axis drives hepatic iron overload, precipitating ferroptotic cell death in ALF. The anti-rheumatoid arthritis drug auranofin restored hepcidin-FPN axis homeostasis and mitigated liver injury, though concomitant upregulation of proinflammatory cytokines limited its therapeutic potential. Strikingly, mesenchymal stromal cells (MSCs) demonstrated superior therapeutic efficacy, coordinately modulating the hepcidin-FPN axis while suppressing ferroptosis through PI3K/Akt/Nrf2 pathway activation. Our findings not only establish the causal relationship between hepcidin-FPN axis dysfunction and ferroptosis-driven liver injury, but also propose MSC-based therapy as a multifaceted strategy targeting both iron homeostasis and ferroptosis for ALF management.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/40393152/
3D cultured human umbilical cord mesenchymal stem cell spheroids regulate oxidative stress and iron homeostasis through the Nrf2 pathway to resist ferroptosis in ovarian granulosa cells and ovarian dysfunction
Oxidative stress-induced death of ovarian granulosa cells (GCs) is a major driver of ovarian functional disorders associated with follicular atresia. Ferroptosis is a key factor in the onset and progression of various ovarian oxidative stress-related diseases, making it a potential target for enhancing reproductive health. Recently, 3D cultured human umbilical cord mesenchymal stem cells (3D hUCMSCs) spheroids have exhibited promising advantages in protecting GCs from oxidative damage. However, it is unclear whether they represent a viable therapeutic strategy for mitigating reproductive failure associated with abnormal follicular atresia by modulating ferroptosis. This study demonstrated that 3D hUCMSC spheroids can effectively protect GCs from hydrogen peroxide (H2O2)-induced oxidative stress and ferroptosis. Additionally, iron overload and lipid peroxidation are two essential features of ferroptosis. 3D hUCMSC spheroids effectively regulate iron uptake and storage to mitigate H2O2-induced iron overload. Furthermore, 3D hUCMSC spheroids mitigate lipid peroxidation induced by H2O2 by restoring GSH metabolic balance and preventing GPX4 inactivation. Mechanistically, the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway was significantly activated by 3D hUCMSCs spheroid treatment. Our findings reveal that Nrf2 knockdown inhibited the 3D hUCMSC spheroids-mediated resistance of GCs to H2O2-induced ferroptosis, and Nrf2 knockdown led to increased iron uptake, resulting in substantial lipid peroxidation through the Fenton reaction, thereby making GCs more susceptible to ferroptosis. This process may involve the ROS-Nrf2-Fe2+ cycle. Significantly, 3D hUCMSC spheroids can mitigate H2O2-induced ferroptosis in GCs by regulating the ROS-Nrf2-Fe2+ cycle. Finally, we confirmed the above results that 3D hUCMSC spheroids ameliorate ovarian oxidative damage in premature ovarian failure (POF) rats. In conclusion, we demonstrated that 3D hUCMSC spheroids regulate oxidative stress and iron homeostasis through the Nrf2 pathway, thereby providing a potential therapeutic target for anovulatory disorders.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/40383404/
Repurposed Nrf2 activator dimethyl fumarate rescues muscle inflammation and fibrosis in an aggravated mdx mouse model of Duchenne muscular dystrophy
In inherited neuromuscular disease, Duchenne muscular dystrophy (DMD), glucocorticoids significantly slow disease progression yet impart side effects severe enough to preclude use in a significant proportion of patients. Extending our findings that acute treatment with FDA approved multiple sclerosis drug, dimethyl fumarate (DMF), rescues muscle pathology in juvenile mdx mice, we aimed to conduct tiered pre-clinical testing toward translation. To aggravate disease phenotype in adult mdx muscles that usually lack human equivalent muscle pathology, we used bi-weekly treadmill running for 4 weeks which increased plasma DMD biomarker, creatine kinase, by 2-fold and quadriceps fibrosis by ∼30 %. Using this model, we screened DMF for 5 weeks in a head-to-head comparison, and in combination, with standard-of-care prednisone (PRED), to model the most likely clinical trial scenario. We show comparable efficacy between DMF and PRED at reducing inflammation via NF-κB suppression and CD68+ macrophage infiltration. Moderate term DMF monotherapy had additional anti-fibrotic and anti-lipogenic effects on skeletal and cardiac muscle beyond those seen with PRED treatment, although combination therapy exacerbated fibrosis in quadriceps. Our study supports DMF as a repurposing candidate for DMD, especially for patients who cannot tolerate chronic glucocorticoid treatment. We also highlight the importance of evaluating combination therapy to identify potential off-target effects between emerging therapeutics and glucocorticoids towards better designed clinical trials.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/40381228/
A self-assembled multicomponent RNA nano-biopesticide for increasing the susceptibility of destructive bean flower thrips to insecticides via dsNrf2
High resistance of bean flower thrips (BFT, Megalurothrips usitatus) has led to the unscientific application of insecticides to cause famous “toxic cowpea” incidents in China. Nuclear factor erythroid 2-related factor 2 (Nrf2) plays an important role in inducing antioxidant responses and drug detoxification. Therefore, the detoxification genes may be suppressed to control insecticide resistance via Nrf2. Herein, we demonstrated that the expression of most detoxification genes and enzyme activity were remarkably suppressed via nrf2 RNAi. Subsequently, a novel hydrophilic-lipophilic diblock polymer (HLDP) was developed to co-assemble with dsNrf2 and sulfoxaflor (SUL) into nanoscale SUL/HLDP/dsNrf2 complex (221.52 nm). Excitingly, the SUL/HLDP/dsNrf2 complex exhibited excellent leaf adhesion performance, with the smaller contact angle, reduced surface tension, amplified contact area, improved retention, and enhanced plant uptake. Meanwhile, theSUL/HLDP/dsNrf2 displayed high delivery efficiency in vitro and in vivo, and its insecticidal activity against BFTs was significantly higher than SUL. Furthermore, the required doses of SUL/HLDP/dsNrf2 to achieve similar insecticidal activity were 50.14% and 58.42% of SUL via immersion and oral feeding, respectively. Overall, this study elucidated the regulatory role of nrf2 in the detoxification and metabolism of BFTs and developed a self-assembled multicomponent RNA nano-biopesticide to increase the susceptibility of BFTs to insecticides.Access to the original article: https://pubmed.ncbi.nlm.nih.gov/40394563/
Polybrominated biphenyls induce liver injury by disrupting the KEAP1/Nrf2/SLC7A11 axis leading to impaired GSH synthesis and ferroptosis in hepatocytes
Polybrominated biphenyls (PBBs) are persistent organic pollutants (POPs) widespread in the environment, presenting significant health hazards due to their bioaccumulation, particularly in liver. Ferroptosis, an iron-dependent form of cell death, has not been previously linked to PBBs-induced hepatotoxicity. This study investigated whether PBBs induce hepatotoxicity through ferroptosis and the toxicological mechanism using mice and THLE-2 cells models exposed to PBB mixture (BP-6). Histopathological and biochemical analyses revealed that BP-6 exposure-induced hepatic injury, oxidative stress, and inflammatory response in mice. BP-6 exposure induced a significant increase in Fe2+ content and a decrease in FTH1, SLC7A11 and GPX4 protein expression in hepatocytes, resulting in severe lipid peroxide accumulation and GSH depletion. Ferroptosis inhibitors, Fer-1 and DFO, reversed the iron metabolism disruption caused by BP-6, underscoring the critical role of ferroptosis in BP-6-induced liver injury. Mechanistically, BP-6 exposure impaired GSH synthesis by preventing Nrf2 nuclear translocation and Slc7a11 transcription through upregulating KEAP1 levels. Keap1 knockdown or Slc7a11 overexpression reversed BP-6-induced lipid peroxide accumulation and GSH depletion, confirming the involvement of ferroptosis in BP-6-induced hepatotoxicity. In addition, curcumin, a natural Nrf2 agonist, significantly alleviated BP-6-induced ferroptosis and liver injury in vitro and in vivo by restoring SLC7A11 protein expression and GSH synthesis. These findings elucidate the toxicological mechanism of PBBs and suggest potential therapeutic strategies to counteract PBBs exposure.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/39934342/
Protective effect of empagliflozin against paracetamol-induced acute kidney injury through modulation of AMPK/SIRT1/PGC-1α pathway in experimental mice
Emerging evidences about paracetamol-induced kidney injury in clinical settings are concerning, especially when administered at high doses. Empagliflozin, an oral SGLT2 inhibitor, employed in the management of diabetes mellitus, exhibits antioxidant, anti-inflammatory, and anti-apoptotic attributes. Thus, the objective of this study is to investigate whether empagliflozin may alleviate paracetamol-triggered nephrotoxicity and unravel the mechanistic insights responsible for its protective impact. In this regard, male mice were assigned to four groups: normal, paracetamol, empagliflozin 10, and empagliflozin 20. Kidney function tests, histopathological examination, immunohistochemistry, oxidative stress biomarkers, inflammatory cytokines, and other molecular targets were detected. Our results showed that paracetamol administration impaired kidney functions along with causing aberrations in renal histoarchitecture. Additionally, paracetamol triggered oxidative stress, inflammation, and apoptosis via hindering the AMPK/SIRT1/PGC-1α cascade and Nrf2/HO-1 while activating the NF-κB hub. Nevertheless, pretreatment with empagliflozin markedly enhanced the kidney function tests and mitigated histopathological alterations caused by paracetamol. Additionally, empagliflozin suppressed the oxidative stress as confirmed by an upregulation of Nrf2, which subsequently increased HO-1, SOD, and GSH, while reducing the MDA level. Moreover, it inhibited the NF-κB-mediated inflammatory process by dampening NF-κB, IL-1β, and TNF-α expressions as well as lowering Bax expression-induced apoptosis. The observed safeguards effects were facilitated via boosting AMPK/SIRT1/PGC-1α signaling trajectory. Collectively, our study verified the enduring reno-protective potential of empagliflozin, particularly at high dose, in the context of paracetamol-induced renal injury by instigating the AMPK/SIRT1/PGC-1α hinge.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/40349789/
Protective factors against oxidative stress in COPD: focus on Nrf2-dependent antioxidant gene expression
Chronic obstructive pulmonary disease (COPD) continues to be the world’s primary cause of morbidity and mortality. The main mechanism driving the pathogenesis of COPD is oxidative stress. Antioxidant genes, regulated by the Nrf2/ARE signaling pathway, play a protective role against oxidative stress. Unfortunately, this pathway appears to be dysregulated in COPD, leading to decreased expression of antioxidant genes and persistent oxidative stress. We reviewed numerous studies measuring the expression of antioxidant genes in COPD. We also focused on developments in methods used to study gene expression in COPD over time, along with measuring antioxidant gene expression in various cell types, and the potential use of antioxidant gene expression as a predictor of COPD progression. And last but not least we discussed the association of cigarette smoke exposure with antioxidant gene expression together with antioxidant treatment in COPD. Understanding the altered expression of antioxidant genes in COPD could help in treating COPD, as well as predicting its progression.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/40385585/
Dimethyl fumarate abrogates hepatocellular carcinoma growth by inhibiting Nrf2/Bcl-xL axis and enhances sorafenib’s efficacy
Hepatocellular carcinoma (HCC) is characterized by poor prognosis and remains a leading cause of cancer mortality worldwide. Advanced HCC is managed with several first-line therapies, including tyrosine kinase inhibitors (TKI) and immunotherapy (mAb-PD-1 and mAb-VEGF). However, the efficacy of HCC therapeutics is often short-lived. Recent studies have demonstrated that the activation of the Nrf2-Bcl-xL pathway contributes to poor prognosis in a subset of HCC patients. Here, we found that dimethyl fumarate (DMF), a drug used for treating psoriasis and multiple sclerosis, regulates the Nrf2-Bcl-xL signaling axis to inhibit HCC growth in a mice xenograft model. Mechanistically, the downregulation of the Nrf2-Bcl-xL axis led to mitochondria stress and apoptosis in vitro and in vivo. Enforced Nrf2 or Bcl-xL expression in HCC cells markedly reversed the antitumor effects of DMF in HCC cells. Importantly, DMF enhanced sorafenib’s antitumor effects. Collectively, our results demonstrate new mechanism insights into the antitumor effects of DMF and that Nrf2-targeted therapy might improve HCC treatment outcomes.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/40369009/
Joana Miranda
Faculty of Pharmacy, University of Lisbon
Portugal

