NRF2 in COST Action 20121
Our quarterly newsletter attempts to provide not only our latest news but also aims at becoming a forum for analysis of relevant topics on the field of NRF2 and provide comments to some of the most relevant articles published during the quarter. You can access our first newsletter comprising November, December 2021, and January 2022 at: https://benbedphar.org/our-first-newsletter/. The following paragraphs summarize the most relevant activities conducted during the last three months.
Scientific meetings. On January 10-11, 2022, we had our first scientific meeting, with 44 speakers and over 100 participants. The meeting gathered some of the most prestigious researches in the NRF2 field from EU, USA and Japan, and presented state of the art work on NRF2 from basic mechanisms to clinical translations in several diseases. You can access the programme and the abstract book at: https://benbedphar.org/benbedphar-online-scientific-marathon/
The next scientific meeting is scheduled for May 19-20. This time it will be face-to-face and will provide an excellent ground to develop friendship as a means to strengthen scientific collaborations and applications to international grants. External participants are welcome and you can see the scientific programme and registration at: https://benbedphar.org/2nd-bedbenphar-scientific-meeting/.
Webinars. In collaboration with the Sapienza University of Rome, we have been organizing a webinar series on biochemical, pharmacological and clinical aspects of NRF2. Our first webinar took place in March 3rd on “NRF2 and cancer”, with three outstanding lectures (Hozumi Motohashi, Donna Zhang, and Gina DeNicola) (https://benbedphar.org/first-webinar/). The second webinar took place on April 26 on “NRF2 and vascular disease”, with the leading experts (Andreas Daiber, Anna Grochot- Przęczek and Giovanni Mann). Both webinars gathered over 100 participants (https://benbedphar.org/webinar-nrf2-and-vascular-diseases%ef%bf%bc/). The next webinar is scheduled for end of June.
Publication in Special issues. At this time we have three special issues being hosted by BenBedPhar: 1) Bench to bedside transition for pharmacological regulation of NRF2 in non-communicable diseases, in Free Radials Biology and Medicine, 2) Transcription Factor NRF2, in Antioxidants, and 3) DNA Damage, Oxidative Stress and Human Disease, in International Journal of Molecular Sciences.
Gender equality. Following the commemoration of the “Girls in Science” and “Working Women” days, our Gender Equality officer, Dr. Elena Milanesi, provided some thoughts about this issue and its relationship with work and family conciliation. In BenBedPhar, the management committee is composed by 54.7% female researchers that were elected due to their exquisite scientific and management capabilities. Read the whole article at https://benbedphar.org/gender-equality-in-benbedphar/
Antonio Cuadrado
Chair of COST Action 20121, BenBedPhar
Autonomous University of Madrid
Comments from the Working groups
Nrf2 keeps up with time
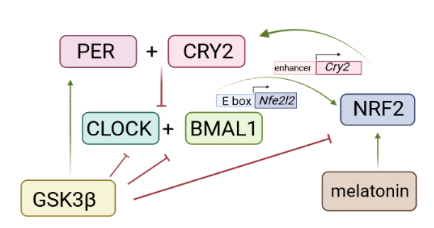
Figure 1. Interplay between the molecular clock and Nrf2 (figure created by Biorender)
The molecular clock allows organisms to prepare and respond to environmental changes throughout the day. Circadian rhythms affect multiple processes, including intracellular redox potential, metabolism or immune responses. That said, it is not surprising that Nrf2 and its triggered homeostatic response are good friends with the biological clock at the cellular and organismal level. Nfe2l2 transcription itself is cycling with a period length of approximately 24 h. The gene contains an E-box element in its promoter and is subject to regulation by BMAL1 and CLOCK, the core circadian clock activating proteins in cells. NRF2 can feedback and reinforce cycling amplitudes by binding to specific enhancer regions of the core clock repressor gene Cry2, increasing Cry2 expression and repressing CLOCK/BMAL1- regulated E-box transcription. NRF2 and the biological clock furthermore share common upstream regulators: Phosphorylation by GSK3β, a major control hub in the non-canonical βTRCP1- mediated degradation of NRF2, leads to degradation of BMAL1 and CLOCK, and activation and nuclear translocation of PER and REV-ERBα. Moreover, melatonin, a pineal gland hormone secreted upon light exposure and a key humoral component of the central circadian clock, leads to activation of the NRF2 signaling pathway.
This interlocking loop between stress response and circadian timing possesses relevant implications for various (patho)physiological settings with very likely many more to come in the future. For instance, IL1β expression in myeloid cells is controlled by the molecular clock via BMAL1/NRF2-driven transcriptional activity and antioxidant capacity, and oscillating BDNF levels mediate NRF2 activation in astrocytes, which in turn can lead to increased survival of dopaminergic neurons. A recent study even brought forward seasonal changes of NRF2 activity in the context of winter depression. Hence, proper timing of NRF2 activation to alleviate or prevent a given condition might be an important point to consider in potential NRF2-targeted pharmacotherapy in order to reach optimal efficacy. When it comes to NRF2, let’s mind the clock and be in time!
Elke Heiss
WG1 member
University of Vienna, Austria
Monitoring target engagement by KEAP1:NRF2 protein-protein interaction inhibitors
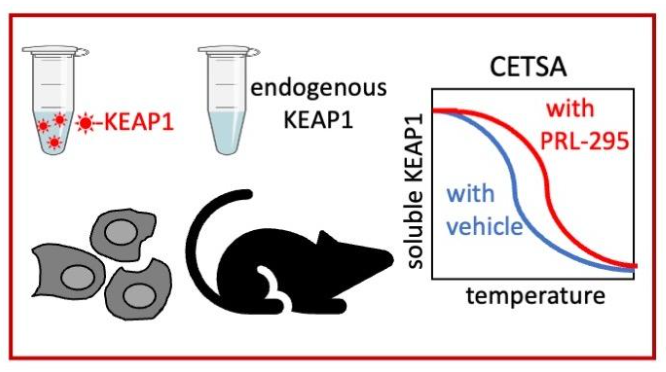
Figure 2: The KEAP1:NRF2 protein-protein interaction (PPI) inhibitor PRL increases the thermostability of KEAP1 in cell lysates, intact cells and in the mouse liver. Figure from: Dayalan Naidu et al, 2021.
The ability to monitor engagement of an intended protein target by a pharmacological agent in the cellular environment is important for target validation in drug development. One way of achieving this is by use of the cellular thermal shift assay (CETSA), as described by Jafari et al (2014, Nat Protoc 9: 2100-2122). CETSA is an assay that detects changes of the thermostability of the target protein upon binding of a small molecule following exposure to increasing temperatures. The levels of the protein in the soluble fraction at each temperature is then quantified, and a temperature-induced aggregation (Tm) curve is generated. CETSA can be coupled with mass spectrometry (CETSA-MS) to allow for evaluation of small molecule-induced changes in the thermal stability of numerous proteins simultaneously (Chernobrovkin et al, 2021, SLAS Discov 26, 534-546). Using CETSA, Dayalan Naidu and their colleagues (2021, iScience 25, 103703.) have recently shown that the isoquinoline PRL-295, a metabolically stable KEAP1:NRF2 protein-protein interaction (PPI) inhibitor (Lazzara et al, 2020, J Med Chem 63, 6547-6560), increases the thermostability of KEAP1 in the physiological environment of the cell (Dayalan Naidu et al, 2021). For this purpose, the temperature-induced aggregation of KEAP1 was assessed following treatment with vehicle or PRL-295. Several experimental systems were tested: (i) lysates from cells expressing either endogenous KEAP1 or stably-integrated fluorescently-tagged overexpressed KEAP1; (ii) intact cells with endogenous KEAP1 expression; (iii) fresh-frozen liver tissues from mice that had been administered orally PRL-295 or vehicle treatments (Figure 1). In all cases, PRL-295 treatment increased the thermostability of KEAP1, but not of HSP90, which was used as a negative control protein. Detailed protocols for these assays, which have been named KEAP1-CETSA and KEAP1-Glow CETSA, will be published in an upcoming issue of STAR Protocols.
Albena Dinkova-Kostova
WG2 co-leader
University of Dundee, UK
Healthy habits help maintaining NRF2 levels
Figure 3. Lifestyle habits such as exercise might induce a mild NRF2 activation leading to reinforcement of homeostatic responses.
Chronic exposure to harmful drivers of disease, in combination with genetic predisposition, can lead to long-term low grade systemic oxidative, inflammatory and metabolic stress which are the most significant underlying hallmarks of non-communicable diseases (NCD) and accelerated ageing. The transcription factor NRF2, as an intracellular integrator and regulator of many homeostatic signals, has recently been acknowledged as one of the major anti-inflammatory and anti-oxidant mechanisms through life. Activators of NRF2 signaling have shown convincing pre-clinical benefits. This rapidly increasing knowledge, coming from genetics and preclinical studies in the NRF2 field, will result in novel strategies for pharmaceutical interventions in NCDs.
However, we can do more… Lifestyle interventions which are based on a balanced diet and an adequate amount of physical activity are considered very successful strategies for restoring metabolic health as well as reducing low grade oxidative and inflammatory stress. Indeed, recent studies have shown that NRF2 can be moderately activated as a mechanism of protection against elevated reactive oxygen species that are generated by physical exercise. In turn this moderated increase might exert hormetic effects through NRF2, providing greater resistance to environmental aggressions, better tolerance to constant changes, and increasing the regenerative capacity. A very exciting and challenging path leads now to lifestyle interventions as a healthy method to fine tune NRF2 activity along lifespan.
Christina Morgernstern
WG3 Co-Leader
Karl-Franzens Universität Graz. Austria
Antonio Cuadrado
WG3 member
Autonomous University of Madrid, Spain
Clinical Trials Assessing Nrf2 as Therapeutic Target
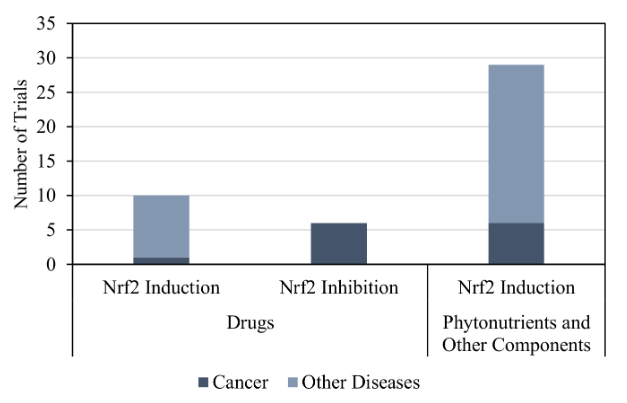
Figure 4. Number of trials that assess the therapeutic activity of drugs and other components in NRF2.
Being a major regulator of antioxidant enzymes and cytoprotective genes, NRF2 has been studied as a therapeutic target in the treatment and prevention of several illnesses (Egea et al., 2019, Front Pharmacol, 10:1149). In the clinicaltrials.gov repository there are 45 trials registered before March 2022 dedicated to the study of Nrf2 as a therapeutic target. In these trials, 16 evaluate drugs and 29 evaluate natural-occurring compounds, such as phytonutrients (curcumin, resveratrol, sulforaphane), peptides, hormones, and vitamins. Diseases under study include chronic (chronic kidney disease, chronic obstructive pulmonary disease), autoimmune (antiphospholipid syndrome, rheumatoid arthritis), neurodegenerative (Friedreich’s ataxia, multiple sclerosis, Alzheimer’s disease), cardiovascular (atherosclerosis, carotid stenosis) and cancers diseases (acute myeloid leukemia, lung, bladder, breast, and rectal cancer). Cancer is the most studied disease, with 7 trials evaluating drugs and 6 evaluating natural-occurring compounds. Phytonutrients (29 trials) and drugs (10 trials) act as NRF2 inducers, but drugs (6 trials) can also act as inhibitors in the treatment of some cancers (Figure 1) that show constitutive activation of NRF2 caused by mutations, which lead to tumour progression and metastases (Zhao et al., 2019, J Cell Mol Med. 23:3451-3463). In conclusion, there is a reduced number of trials that evaluated drug activity in NRF2 compared to the trials that assessed the activity of phytonutrients and other components. In addition, the inhibition of NRF2 in the treatment of cancer starts to gain relevance.
Marisa Antunes
Fernando Antunes
co-leader of WG4
University of Lisbon, Portugal
Much more than an antioxidant-response regulator. NRF2 at the center of muscle homeostasis
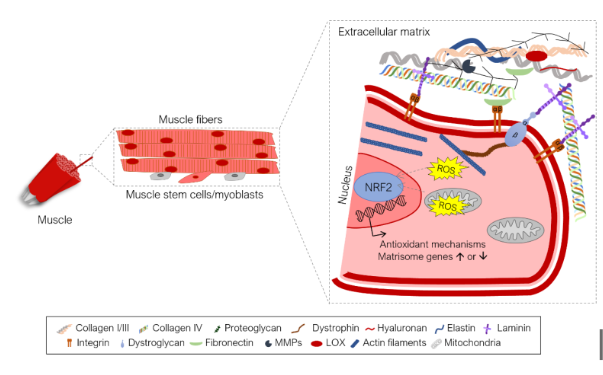
Figure 5. NRF2 role in muscle tissue remodeling and repair.
NRF2 was first discovered in 1994 and since then the research on this transcription factor never stopped. While classically viewed as a master regulator of the antioxidant response, soon it became evident that NRF2 also regulated several other pathways including, cell proliferation and survival, DNA repair, autophagy, mitochondrial biogenesis, cell metabolism, extracellular matrix (ECM) remodeling or anti-inflammatory response. Moreover, the cues that lead to NRF2 activation are not limited to increased oxidative stress levels, but involve many other insults, such as metabolic stress, oncogene induction, DNA damage, hypoxia or tissue damage. Not knowing that this article is centered on NRF2, this description could well be used to define p53, a famous transcription factor often defined as the “guardian of the genome”. This certainly highlights the importance of NRF2 to maintain cell homeostasis and therefore is not surprising to see changes in NRF2 expression associated to a vast range of diseases.
Let’s focus for example on muscular dystrophies, a set of conditions frequently associated to mutations in genes coding for ECM components or components that link the ECM to the cell cytoskeleton, and which are commonly characterized by hypotonia and muscle loss. In addition, increased levels of oxidative stress have been shown to play an important role in the pathology of muscular dystrophies. In this case, NRF2 has been pointed out as a potential therapeutic target and its activation may ameliorate disease pathology, for example by promoting an antioxidant response, enforcing mitochondrial stability or enhancing muscle stem cell activity (Kourakis et al., 2021, Redox Biol. 38: 101803). But the role of NRF2 in the response to disease pathology in muscular dystrophies may be even more direct, since NRF2 regulates the transcription of 138 matrisome genes, i.e., genes that encode not only ECM components but also ECM remodeling enzymes, ECM-associated factors and secreted proteins (Hiebert, 2021, Matrix Biol. Plus, 10: 100057; Martins et al., 2021, Front. Genet. 12: 1279). Therefore, NRF2 is emerging not only as regulator of the antioxidant-response in the muscle, but also as a key factor, which directly guides tissue remodeling, by regulating the transcription of several matrisome genes. This poorly explored role of NRF2 in tissue remodeling, may be a key function of NRF2 countering tissue damage in several pathologies.
Ana Rita Carlos
WG5 Co-Leader
University of Lisbon, Portugal
Hot from Pubmed
Plant-derived NRF2 activators as complementary treatment for COVID‐19.
Although vaccination remains the main preventive measure against COVID‐19, the identification of new cost‐effective treatments represents an important challenge. In this scenario, it has been proposed that specific plant‐derived nutrients may play a role in preventing or controlling respiratory tract infections, including COVID‐19. Accumulating data have demonstrated that curcumin, a phytochemical extracted from Curcuma longa rhizomes, stimulates the NRF2 signaling pathway. Recent preliminary clinical trials, suggested that the use of this natural product could be a complementary treatment to prevent some of the COVID-19 symptoms, to reduce morbidity and to shorten the duration of sick leave.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35344297/
Synthetic triterpenoid derivatives as KEAP1/BACH1 inhibitors.
Synthetic triterpenoids facilitate antioxidant response and reduce inflammation in several models, and their action are widely attributed to their ability to activate NRF2. Since the transcription negative regulator BACH1 is a repressor of HMOX1, which is positively regulated by the transcription factor NRF2, it is considered a potential therapeutic target in cancer. A recent study carried on at University of Dundee, UK, found that two synthetic CCDO triterpenoid derivatives, CDDO-Me and CDDO-TFEA, target the transcriptional repressor BACH1 and activate NRF2. Moreover, it has been shown that both compounds impaired lung cancer cell invasion in a BACH1-dependent and NRF2-independent manner.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35313207/
Omaveloxolone as a promising NRF2 activator for intracerebral hemorrhage treatment.
Omaveloxolone (Omav), a second-generation member of the synthetic oleanane triterpenoid compounds, has been demonstrated to improve the neurological function of patients with Friedreich ataxia by regulating the antioxidant response. A study conducted at Zhejiang University School of Medicine, China, investigated the efficacy of this compound in intracerebral hemorrhage (ICH). Through in vitro and in vivo experiments, it was demonstrated that Omav inhibited M1-like activation of microglia, whilst promoting the M2-like phenotype. Notably, the administration of this drug to ICH mice was able to improve their sensorimotor function, while the use of a selective NRF2 inhibitor repressed this function. This study showed that Omav modulated microglial polarization by activating NRF2 and inhibiting ROS generation in ICH models indicating that it is a promising drug for the treatment of ICH.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35308167/
Pericytes stemness acquisition via NRF2.
Pericytes are regarded as multipotent cells, activated in response to tissue injury thus contributing to the regenerative process. A research group from Kwansei Gakuin University, in collaboration with Multi-Modal microstructure analysis unit and the laboratory for cellular function imaging of the RIKEN Center from Japan, recently isolated normal brain pericytes (nPCs) from the cortex of a mouse model of ischemic stroke. Since it has been proposed that nPCs are converted to ischemic pericytes (iPCs) the group compared the traits of the two cell populations, demonstrating that oxidative stress and NRF2 are necessary for the generation of stem cells after stroke.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35353891/
NRF2 deficiency and attenuation of atherosclerosis signs.
Oxidative stress represents a major cause of endothelial dysfunction and abnormal proliferation and migration of vascular smooth muscle cells (VSMCs). These two conditions are involved in atherosclerosis onset and progression. Investigating an atherosclerosis mice model, Hongliang Li and collab. found that the aorta and VSMCs of these mice had increased NRF2 levels compared with the controls. NRF2 deficiency in Apoe-/-Nrf2-/- mice attenuated the atherosclerotic plaque burden, diminished proliferation, and migration of VSMCs. Importantly, using cultured mouse VSMCs to study the interaction between NRF2 and the promoter sequence of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), the researchers revealed that NRF2 insufficiency can reduce the pathological process by blunting LOX-1-mediated proliferation and migration of VSMCs.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35299056/
NRF1 and NRF2 in ferroptosis resistance.
The involvement of the Cap’n’collar (CNC) transcription factors NRF1 and NRF2 in regulating the iron-dependent oxidative death by ferroptosis was investigated. NRF2 has been shown to inhibit ferroptosis by over-expression of the SLC7A11 gene which encodes a cystine/glutamate antiporter subunit, as well as of the GCLC and GCLM genes involved in glutathione biosynthesis. Meanwhile, NRF1 was shown to act through a different mechanism. In this case, ferroptosis is inhibited through an increase of NRF1 stability due to its deglycosylation by cytosolic peptide, N-glycanase 1, leading to sustained expression of glutathione peroxidase 4 (GPX4), which further prevents lipid peroxidation. These mechanisms can complement each other for promoting ferroptosis resistance in cancer cells, hence limiting the efficacy of anti-tumor therapies based on oxidative stress.
Access to the original article: https://doi.org/10.1073/pnas.2118646119
NRF2, IDO1 and kynurenin connection in ferroptosis resistance.
A new link between indoleamine 2,3-dioxygenase (IDO1), cell survival mediated by kynurenines (KYN) and ferroptosis resistance was emphasized in cancer. In IDO1-expressing cells, KYN, generated through IDO1-mediated oxidation of tryptophan, is released and is further uptaken by neighbouring non-IDO1 cells via the SLC7A11 antiporter. In both types of cells, KYN is converted into downstream metabolites that suppress ferroptosis by scavenging ROS and by activating a NRF2-dependent, AHR-independent cytoprotective pathway, including the NRF2 target SLC7A11, for propagating anti-ferroptotic signals. These are new arguments for the use of IDO1 inhibitors in cancer treatment, aiming to down-regulate the multi-pronged protection pathways against ferroptosis in cancer cells.
Access to the original article: https://doi.org/10.1016/j.molcel.2022.02.007
KEAP1-EMSY – a critical pathway in non-small cell lung cancer.
The authors show that KEAP1 targets the BRCA2-interacting protein EMSY for ubiquitin-mediated degradation, aiming to regulate genome stability through homologous recombination repair (HRR) in non-small cell lung cancer (NSCLC). Therefore, KEAP1 loss-of-function mutations induce EMSY stabilization and consequent HRR defects that contribute to a high mutational burden in tumors. Moreover, EMSY accumulation suppresses the type I interferon response in the tumor microenvironment, leading to impaired innate immunity that accounts for the immune evasion of tumors. Therefore, authors suggest that the Poly (ADP-ribose) Polymerase (PARP) and STING signaling pathways might be therapeutically targeted in NSCLC harboring KEAP1 alterations, for restoring HRR defects and anti-tumor immunity, respectively.
Access to the original article: 10.1016/j.cell.2021.12.005
The NRF2-KEAP1 pathway in oncology
The KEAP1/NRF2 pathway plays a physiologic protective role against xenobiotics and reactive oxygen species; while activation of NRF2 gives selective advantage for tumors by rewiring metabolism to enhance proliferation, suppress various forms of stress, and promote immune evasion.The review critically revises the current experimental and clinical knowledge on the role of the NRF2-KEAP1 pathway in oncologic pathologies, addressing (epi)genetic alterations, transcriptional regulation and post-transcriptional modifications that induce metabolic rewiring of tumors in terms of redox and amino acids status as well as heme and iron metabolism, among other disturbances. Besides describing the mechanism underlying the complex NRF2 contribution to oncogenesis and cancer progression, the authors address its involvement in tumor resistance to radio- and chemo-therapies. Special emphasis is given to the impact of the NRF2-charged microenvironment on anti-tumor immunity, as a new line for therapeutic intervention. For instance, the KEAPSAKE trial, combining the glutaminase inhibitor telaglenastat with standard first-line therapy in patients with tumors that carry loss-of-function mutations in KEAP1 or activating mutations in NRF2, has shown that the combined therapy can reduce tumor-intrinsic metabolic vulnerabilities and augments also the anti-tumor response of T cells. Authors comment that, besides addressing metabolic disturbances in NRF2-addicted tumors, there is a need to target NRF2 signaling in itself, in the context of multiple and aggressive co-occurring mutations in tumors.
Access to the original article: 10.1158/2159-8290.CD-21-0922
Gina Manda
Vice-Chair
Elena Milanesi
Gender Equality Officer
Victor BabesNational Institute of Pathology, Romania
Joana Miranda
University of Lisbon, Portugal


