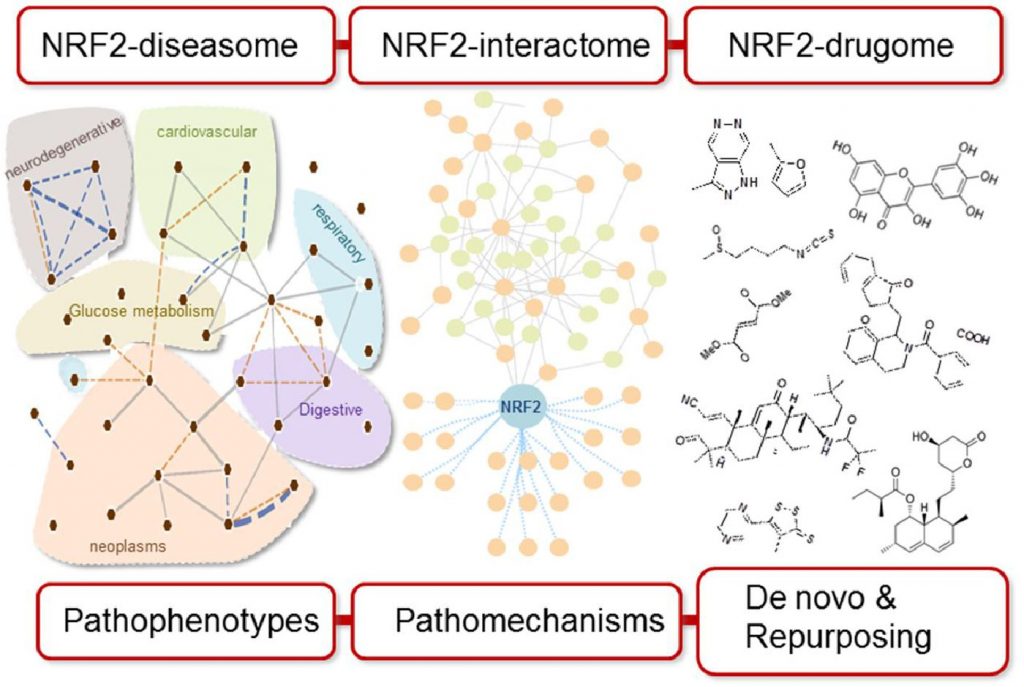
January 10
- 11:00 - 11:30 Welcome message and general information. Antonio Cuadrado
- 11:30 - 13:00 SESION 1. Chair: Anna Grochot-Przęczek
NRF2-CEBPB cooperativity in transcriptional regulation
Hozumi Motohashi
Department of Gene Expression Regulation, Institute of Development, Aging and Cancer, Tohoku University
Email: hozumim@med.tohoku.ac.jp
NRF2 is a master transcription regulator that coordinately regulates many cytoprotective genes and plays a central role in defense mechanisms against oxidative and electrophilic insults. While increased NRF2 activity is principally beneficial for our health, outcome of NRF2 activation in cancer cells is detrimental which is observed in almost 15% of non-small cell lung cancer (NSCLC). We conducted an unbiased approach by investigating NRF2-dependent transcriptome in NSCLC cell lines with NRF2-activated NSCLCs, and in those with NRF2-normal NSCLCs. We identified a battery of genes that are regulated by NRF2 specifically in NRF2-activated NSCLCs and found that these genes are accompanied by unique NRF2-dependent enhancers. CEBPB accumulation in NRF2-activated NSCLCs is found to be one of the prerequisites for the establishment of the unique enhancers, in which NOTCH3 enhancer is critical for the promotion of tumor-initiating activity. To understand NRF2-CEBPB cooperativity in more detail in NRF2-activated NSCLCs, we comprehensively explored NRF2-CEBPB-coregulated genes by comparing the NRF2- and CEBPB-dependent transcriptomes in NRF2-activated NSCLC cell lines. Genes involved in drug metabolism and detoxification were found to be enriched in the coregulated genes accompanied by NRF2-CEBPB-coregulated enhancers. These results suggested that enhanced activities of stem-like phenotype, drug metabolism and detoxification are achieved by the cooperative function of NRF2 and CEBPB in NRF2-activated NSCLCs.

Hozumi Motohashi is a full professor at the Department of Gene Expression Regulation, Institute of Development, Aging and Cancer, Tohoku University. After identifying CNC-small Maf heterodimers as a new transcription factor family members, she is focusing on NRF2 roles in various pathological conditions, especially in cancers. NRF2 functions and its contribution to cellular homeostasis seem to be altered in cancer cells with persistent activation of NRF2 compared with normal cells with transient activation of NRF2 when it is necessary. She is trying to understand the unique activity of NRF2 in NRF2-activated cancer cells from the viewpoint of epigenetic regulation and metabolic regulation.
Peroxiporins and NRF2
Ana Čipak Gašparović
Laboratory for Oxidative Stress, Division of Molecular Medicine, Rudjer Boskovic Institute, Zagreb, Croatia
Email: acipak@irb.hr
Breast cancer is still one of the leading cause of morality in women. Despite of all measures for early detection and prevention, there is no significant progress in the survival rates. One of the reasons for this is therapy resistance. Aquaporins, firstly described as cellular plumbing system, provided mechanisms for cancer cell motility and proliferation, and as such are novel targets for cancer treatment. Studies revealed the complexity of mechanisms and involvement of aquaporins in physiological processes in the cell thereby indicating the need to study mechanisms by which specific aquaporins contribute no both, normal and pathological processes. This is especially interesting from the oxidative stress aspect, as some aquaporins, referred as peroxiporins, channel hydrogen peroxide as well, thereby modulating pathways involved in redox signaling. Therefore, the possibilities of peroxiporins having a role in redox signaling pathways, especially NRF2, could provide novel mechanisms in cancer development.
Ana Čipak Gašparović is a Senior Research Associate in the Laboratory for Oxidative Stress, Division of Molecular Medicine at the Rudjer Boskovic Institute in Zagreb, Croatia. Her research focuses on the role of oxidative stress and antioxidative response in the development of the resistance to cancer treatment. Recently, her research included aquaporins in breast and colon cancer. Special emphasis is given to peroxiporins, specific aquaporins which, in addition to water and glycerol, channel hydrogen peroxide, and as a consequence contribute to oxidative and antioxidative response of the cell. She is interested in the regulation of NRF2 pathway in response to peroxiporins, and thier influence on the delvelopment of therapy resistance.
NRF2 activity is impaired in a DPR model of C9orf72-related ALS through non-classical mechanisms.
Ana I Rojo
Department of Biochemistry, Medical School, Autonomous University of Madrid, Spain
Email: airojo@iib.uam.es
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease, characterized by motoneuron demise and muscle denervation, leading to loss of voluntary movements and eventually death. Multiple of the pathways dysregulated in ALS, such as oxidative and inflammatory stress, autophagy and mitochondrial control share a link through the transcription factor Nuclear-related erythroid 2-related 2 (NRF2). An impairment of the NRF2 pathway in ALS has been widely reported. However, the molecular mechanisms governing it have yet to be determined in-depth. In this study, we have analysed NRF2 dysfunction on an in vitro model of dipeptide repeats (DPRs) from C9orf72-related familial ALS. We found that cells overexpressing GFP-DPRs exhibited higher superoxide anion levels than GFP overexpressing counterparts, accompanied by reduced NRF2 accumulation kinetics. This impairment is produced not through a decrease in Nfe2l2 mRNA levels or splicing alterations, but through changes in mRNA stability and translation. In addition, GFP-DPRs impair the induction of a NRF2 activation reporter and a NRF2 transactivation reporter. Indeed, production of DPRs from an inducible cassette bearing the hexanucleotide expansion causative of C9orf72-related ALS led to decreased NRF2 activation and induction of its downstream target heme oxygenase 1 by dimethyl fumarate. Furthermore, we analysed whole blood mRNA samples from ALS patients bearing mutations in the C9orf72 gene. We detected a reduction in the levels of NFE2L2 and its downstream targets NQO1 and HMOX1, in concordance with the changes found in our in vitro models. Therefore, our findings point to a non-classical mechanism governing NRF2 dysfunction in C9orf72-related ALS and reveal concordant changes between ALS models and patients that could be used as novel biomarkers in preclinical and clinical research.

Ana I Rojo is assistant professor of Biochemistry and Molecular Biology at the Department of Biochemistry, Medical School, Autonomous University of Madrid. Her professional career is focused on the study of the molecular basis of neurodegenerative diseases and in the search for novel brain protective therapies. Specifically, she worked on the connection of the progression of Parkinson’s disease, Alzheimer’s disease and more recently Amyotrophic Lateral Sclerosis with the loss of the activity of the transcription factor NRF2, a crucial regulator of multiple responses to stress, whose activity decreases with aging. Her studies demonstrate the relevance of normal homeostatic responses, including NRF2, in protection against proteotoxic, inflammatory and oxidative stress, providing new molecular targets to combat neurodegeneration.
The NRF2-KEAP1 signaling pathway as a pharmacological target in cardiovascular and neuropsychiatric complications in response to environmental risk factors traffic noise and particulate matter
Andreas Daiber
Department of Cardiology 1, Laboratory of Molecular Cardiology, University Medical Center of the Johannes Gutenberg University, Mainz, Germany
Email: daiber@uni-mainz.de
Environmental risk factors, including noise, air pollution, chemical agents, and ultraviolet radiation (UVR) have a considerable impact on human health. Oxidative stress and inflammation arise as key players in molecular pathomechanisms of environmental pollution. There is evidence for protective actions of NRF2 in connection to oxidative stress and inflammation in response to environmental risk factors. We have recently demonstrated the protective actions of NRF2 and its downstream pathways against traffic noise associated cardiovascular damage. Similar evidence can be found in the literature for a beneficial effect of NRF2 against air pollution induced complications as well as detrimental health effects by other environmental stressors, such as UVR, heavy metals and smoking. Question is whether NRF2 activation in the general population by nutraceuticals could be used to prevent non-communicable chronic disease development and progression by environmental stressors.

Andreas Daiber studied Chemistry at the University of Konstanz (Diploma in 1997), holds a PhD in Biochemistry (graduated in 2000), did his habilitation (graduated in 2006), and since 2008 is a full professor in Molecular Cardiology at the University Medical Center Mainz. >33 significant research grants from the pharmaceutical industry and public funding bodies. 2011 guest professorship at the Université Joseph Fourier at Grenoble, France. From 2014-2016 Chair of COST Action BM1203 (EU-ROS). Memberships in national and international scientific communities (SFRBM/SFRRE, ASBMB, DGK), reviewer activities for numerous scientific journals (e.g. FRBM, Redox Biology, ATVB, Eur. Heart J., Nat. Comm.) and funding bodies, editorial board positions (Oxid. Med. Cell. Longev., Cardiovasc. Res., Antioxidants, FRBM, Redox Biology), guest editor (Antioxid. Redox Signal., Redox Biology, Br. J. Pharmacol., FRBM, Antioxidants). He published >170 original research articles, >130 review articles, 25 book chapters, >155 conference abstracts and 2 patents with Boehringer Ingelheim. h-index: 69; >13,000 citations. Special research interests: redox biochemistry, oxidative stress and environmental research in cardiovascular disease.
NRF2 Associated Immune Evasion in Non-Small Cell Lung Cancer and Squamous Malignancies
Anna-Liisa Levonen
A.I.Virtanen Insitute for Molecular Sciences, Faculty of Health Sciences, University of Eastern Finland, Finland
Email: anna-liisa.levonen@uef.fi
The NRF2 pathway is frequently activated in various cancer types, yet a comprehensive analysis of its effects across different malignancies is currently lacking. We developed a robust NRF2 activity metric and utilized it to conduct a pan-cancer wide analysis of oncogenic NRF2 signaling. We identified a distinct immunoevasive phenotype where high NRF2 activity is associated with low interferon-gamma (IFNγ), HLA-I expression and T-cell infiltration spanning non-small cell lung cancer (NSCLC) and squamous malignancies of head and neck area, cervix and esophagus. In squamous cell cancers, NRF2 overactive tumors comprise a molecular phenotype with SOX2/TP63 amplification, TP53 mutation and CDKN2A loss. These immune-cold NRF2 hyperactive diseases are associated with upregulation of immunomodulatory NAMPT, WNT5A, SPP1, SLC7A11 and SLC2A1 that represent candidate NRF2 target genes, suggesting direct modulation of the tumor immune milieu. Based on single-cell mRNA data, coupled with a priori information on intercellular ligand-receptor interactions, cancer cells of this subtype exhibit decreased expression of IFNγ responsive ligands, and increased expression of immunosuppressive ligands NAMPT, SPP1 and WNT5A that mediate signaling in intercellular crosstalk. As we observed differential cytokine mRNA expression with IFNγ treatment in NSCLC adenocarcinoma subtype, we explored the cytokine secretome in vitro. We found that secreted neutrophil chemoattractants interleukin-8 (CXCL8) and ENA-78 (CXCL5) are elevated in NRF2 overactive cells, suggesting contribution of immunosuppressive neutrophils in NRF2 driven immune escape.
Importantly, as overactive NRF2 is associated with immune-cold characteristics, our results
highlight the utility of NRF2 pathway activation for stratifying immune checkpoint blockade responders and non-responders across NSCLC and squamous cancers. Given that in NSCLC NRF2 pathway activation is largely due to somatic mutations in either KEAP1 or NFE2L2, identification of mutations of these genes from cell free DNA may provide a simple and non-invasive method for patient stratification.

Anna-Liisa Levonen is currently Professor and Vice Dean of Research at the University of Eastern Finland, Faculty of Health Sciences. She earned her MD and PhD degrees from the University of Helsinki, Finland in 1994 and 2000, respectively, followed by a fellowship at the University of Alabama at Birmingham (UAB), Department of Pathology and Center for Free Radical Biology. Upon her return to Finland, she was recruited to the University of Kuopio (currently University of Eastern Finland), where she has established a research program focusing on the gene regulatory mechanisms activated by oxidative and electrophilic stress, particularly via the redox-activated transcription factor NRF2. She has studied the mechanism of activation of the KEAP1-NRF2 pathway and its role in disease, particularly cancer and cardiometabolic diseases, and has published highly cited original and review articles on the topic.
NRF2 as a Pharmacological Target in Renal diseases
Juan Antonio Moreno
Department of Cell Biology, Physiology and Immunology, Cordoba University, Spain
Group GE-06 Pathophysiology of renal and vascular damage at Maimonides Biomedical Research Institute of Cordoba (IMIBIC), Spain.
Email: juan.moreno@uco.es
Renal disease is one of the most important public health problems due to its elevated prevalence, high mortality rates, and decreased health-related quality of life. Renal disease may be classified as chronic kidney disease (CKD) and acute kidney injury (AKI). CKD is related to a progressive loss of renal function, leading to dialysis or kidney transplantation AKI refers to a sudden decrease in renal function that may be associated to increased mortality risk. Pathologically, renal disease is related to increased oxidative stress and inflammation. However, therapies to slow or prevent renal disease progression remain an unmet need. NRF2 (nuclear factor erythroid 2-related factor 2) is a transcription factor that plays a key role in protection against oxidative stress and regulation of the inflammatory response. Consequently, the use of compounds targeting NRF2 has generated growing interest for nephrologists. Increased expression of NRF2-regulated genes has been observed in experimental models and human renal biopsies of both CKD and AKI patients. Pre-clinical and clinical studies have demonstrated that NRF2-inducing strategies prevent CKD progression. Moreover, our group has demonstrated that activation of NRF2 may be useful to protect from AKI by decreasing oxidative stress, inflammation and cell death.

Juan Antonio Moreno is a Ramon y Cajal Tenure Track Researcher at Department of Cell Biology, Physiology and Immunology, Cordoba University. He is also chief of the Group GE-06 Pathophysiology of renal and vascular damage at Instituto Maimonides de Investigación Biomédica de Córdoba (IMIBIC). Our group is unravelling novel pathogenic mechanisms involved in the development of renal and vascular diseases. We aim to understand the basis for alterations in renal and vascular wall to identify new molecules involved in the progression of these pathologies that may be used as potential diagnostic/prognosis biomarkers and to develop novel therapeutic approaches. Specifically, we are interested in certain cellular and molecular aspects (oxidation, inflammation, apoptosis, fibrosis, intracellular signalling pathways..) involved in the progression of several pathologies (atherothrombosis, diabetic nephropathy, glomerular diseases, acute kidney injury, renal fibrosis, among others). In the last years, we have evaluated the role of Nrf2 in renal diseases and we are interested in the validation of novel compounds targeting Nrf2 to decrease renal damage.
- 13:00 - 14:00 Breakout rooms and lunch.
- 14:00 - 15:30 SESION 2. Chair: Ana I. Rojo
Novel atypical functions of Nrf2 and Keap1 in endothelial cells
Anna Grochot-Przęczek
Department of Medical Biotechnology, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, Krakow, Poland
Email: anna.grochot-przeczek@uj.edu.pl
A single layer of cells lining the internal surface of blood vessels is the vascular endothelium. The endothelium not only creates a structural barrier between blood and tissues but, most important, is a dynamic and multifunctional endocrine organ. Healthy endothelium is of fundamental significance for vascular homeostasis, and its dysfunction is observed in many cardiovascular diseases (CVD). We found that Nrf2 and Keap1 regulate endothelial cells’ angiogenic response and premature senescence by newly discovered atypical activities. We showed that Nrf2 tethers Keap1 to prevent podosome disassembly, permitting cell migration and angiogenesis. This function of Nrf2 predominates its transcriptional activity in regulating blood vessel formation. Moreover, we identified Keap1 to form together with GAPDH and NOS an S-nitrosation enzymatic complex, which modulates endothelial cells and blood vessels’ premature senescence, affecting cellular proteostasis. Our study shows the critical role of Nrf2/Keap1 in vascular biology and underlines their non-classical activities, reaching beyond gene transactivation and Nrf2 repression.

Anna Grochot-Przęczek is an assistant professor at the Department of Medical Biotechnology, Faculty of Biochemistry, Biophysics and Biotechnology, at Jagiellonian University in Krakow, Poland. She studies the molecular mechanisms regulating the function of endothelial cells and blood vessels. Her team’s research showed that Keap1, GAPDH and nitric oxide synthase (NOS) form an enzymatic complex catalysing protein S-nitrosation. This process is deregulated in aged endothelial cells and blood vessels, which leads to massive protein aggregation. Currently, she tries to recognise the significance of Nrf2/Keap1 imbalance and the Keap1-dependent loss of proteostasis in the function of blood vessels.
The NRF2-KEAP1 Signaling Pathway as a Pharmacological Target in Neurodegenerative diseases
Antonio Cuadrado
Department of Biochemistry, Medical School, Autonomous University of Madrid, Spain
Email: antonio.cuadrado@uam.es
The etiology of Alzheimer’s disease (AD) remains largely unknown and the therapeutic approaches to stop disease progression have consistently failed. This fact indicates that, in addition to the largely studied amyloid and Tau pathophenotypes, other crucial factors must be considered. Our view is that the loss of homeostatic responses in the elderly is a very relevant factor for AD onset and progression. In recent years, a master regulator of homeostatic responses has been found in the transcription factor NRF2 (Nuclear factor E2-Related Factor 2). NRF2 regulates the expression of over 250 homeostatic genes that play crucial roles in protection against oxidative, inflammatory, metabolic, and proteotoxic forms of cellular stress, all of which are well established pathomechanisms of AD and its co-morbidities. In postmortem brain samples of AD patients, neurons expressing aggregates of TAU or APP exhibit increased levels of the NRF2 targets NQO1 and SQSTM1 and in protein lysates we found upregulation of NRF2 in parallel to increased markers of microgliosis, astrogliosis and inflammation. These results suggest that the increase in NRF2 activity found in the patients is a partially unsuccessful attempt of the diseased brain to compensate the pathologic events. Consistent with this hypothesis, reinforcement of the NRF2 signature in mouse models of AD by daily treatment with an NRF2 activator reduced glial and inflammatory markers and improved cognition and motor complications.

Antonio Cuadrado is full professor of Biochemistry and Molecular Biology at the Department of Biochemistry, Medical School, Autonomous University of Madrid. He studes the molecular mechanisms involved in initiation and progression of neurodegenerative diseases. For the past years his main lane of research has been the validation of transcription factor NRF2, master regulator of cellular homeostasis, with four main lines of activity: i) The transcription factor NRF2 as a new therapeutic target in Parkinson’s and Alzheimer’s diseases. ii) Role of oxidative stress in neuronal death and neuroinflammation in neurodegenerative diseases. iii) Pharmacological regulation of autophagy in the brain as a novel therapeutic strategy for neurodegenerative proteinopathies. iv) Development of new NRF2-modulating drugs.
In silico based development of the activators of NRF2-KEAP1 signaling pathway for the treatment of the dermatological disorders.
Arie Gruzman1, Shirin Kahremany1,2, Guy Cohen2
1Department of Chemistry, Faculty of Exact Sciences, Bar-Ilan University, Ramat-Gan, Israel
2Skin Research Institute, Dead Sea & Arava Science Center, Massada, Israel
Email: gruzmaa@biu.ac.il
The Nrf2 signaling pathway plays a pivotal role in neutralizing excess ROS formation and therefore enhancing the endogenous cellular protection mechanism. Thus, activating this pathway may provide therapeutic options against oxidative stress-related disorders. We have recently applied a computer-aided drug design approach to the design and synthesis of novel Nrf2 enhancers. The current study was aimed at investigating the potential beneficial impact of two developed by us molecules: (E)-5-oxo-1-(4-((2,4,6-trihydroxybenzylidene)amino)phenyl)pyrrolidine-3-carboxylic acid (SK-119) and 5-Oxo-1-(4-((2,4,6-Trihydroxybenzylidene)Amino)Phenyl) Pyrrolidine-3-Carboxylic Acid (SH-29) in skin oxidative damage models.
Both compounds were able to attenuate key pathways underlying oxidative stress related damage, including cytosolic and mitochondrial reactive oxygen species (ROS) generation, tested by DC-FDA and MitoSOX fluorescent dye, respectively. This effect was independent of the low direct scavenging ability of the compounds. In addition, both SK-119 and SH-29 were able to reduce oxidative stress-induced IL-8 hypersecretion in pharmacologically relevant concentrations. Lastly, the safety of both compounds was evaluated and demonstrated in the ex vivo human skin organ culture model. These results indicate that SK-119 and SH-29 are Nrf2 activators and might be used as a prototype molecules for the development of novel treatment of dermatological disorders related to oxidative stress. Now, structures of both compounds are serving the ongoing research for for the creation of one lead compound with excelent pharmacokinetic profile for the dermatological applications.

Arie Gruzman is associate professor of medicinal chemistry and pharmacology at Department of Chemistry, the Faculty of Exact Sciences, Bar-Ilan University, Ramat-Gan, Israel and the National Representative of the Chemistry and Human Health Division of IUPAC for the term 2022-2023.
Deciphering the inflammatory role of lung myeloid cells in response to Streptococcus pneumoniae infection
Belén de Andrés and Mª Luisa Gaspar
Immunobiology Department. National Center for Microbiology. Instituto de Salud Carlos III, Majadahonda, Madrid. Spain
e-mail: bdandres@isciii.es; mlgaspar@isciii.es
Bacterial pneumonia is primarily caused by S. pneumoniae, which constitutes a global health problem due to increasing antibiotic resistance and lack of effective vaccines. The initial immune response to bacteria occurs when the innate immune system recognizes common motifs expressed by pathogens, through pattern recognition receptors such as Toll-like receptors (TLRs) expressed by epithelia and immune cells. In the case of S. pneumoniae, the bacterium is mainly recognized by TLR4 and TLR2 expressed on the surface of the cells. We have used a murine model of nasal instillation of S. pneumoniae in wild-type (WT), TLR4 and MyD88 (Innate Immune Signal Transduction Adaptor) deficient mice. Compared to WT mice, there was an increased bacterial load in the two deficient strains, being TLR4-/- mice more susceptible to bacterial infection. Our data demonstrated the rapid recruitment of alveolar macrophages and natural killer cells in the first 24 hours after infection, followed by recruitment of neutrophils, monocytes, and macrophages at 72 hours, all of which were profoundly decreased in the absence of TLR4. The respiratory burst (measured by ROS production) after infection, was produced mainly by neutrophils and monocyte-derived dendritic cells, and to a lesser extent by monocytes and macrophages, all being severely decreased in TLR4-/- mice, and to a lesser extent in MyD88-/- mice. Induction of Nox2 was detected after infection, together with down-regulation of Nrf2 in WT and MyD88-/- but not in TLR4-/-. In this sense, maintaining Nrf2 levels and weaker Nox2 induction may contribute to the diminished ROS production in TLR4-/-infected mice. These data demonstrate the complex myeloid population dynamics and a key role of the TLR4 signaling axis in the response to S. pneumoniae.
Belén de Andrés is senior researcher and Mª Luisa Gaspar is full professor, both at the Instituto de Salud Carlos III, Majadahonda, Madrid. Spain. Our group is interested in the analysis of immune responses of myeloid cells and B lymphocytes after lung infections, focusing on TLR-dependent activation, the inflammatory response and the regulation of the oxidative metabolims (Nrf2, Nox2, NADPH activation pathways). In this regard, our contributions are related to immune cell dynamics and differentiation in the neonatal and aged context, cytokine profiles, oxidative metabolites and immunoglobulin repertoires.
Targeting Intracellular Beta-Amyloid Accumulation: On the path for Preventing Alzheimer’s Disease
Cláudia Guimas Almeida
CEDOC – Chronic Diseases Research Center, NOVA Medical School, Universidade NOVA de Lisboa, Portugal
Email: claudia.almeida@nms.unl.pt
The etiology of late-onset Alzheimer’s disease (AD) is multifactorial, with aging being the biggest risk factor and genetic predisposition accelerating the disease onset. While beta-amyloid accumulation in plaques is a hallmark of neurodegeneration, intracellular beta-amyloid accumulation is the hallmark of early synaptic dysfunction preceding AD onset. Thus, reducing intracellular beta-amyloid should be a target for preventing or delaying AD. In the common late-onset AD, what causes increased intracellular beta-amyloid accumulation remains to be established. Since beta-amyloid is produced intracellularly upon APP processing in endosomes and controlled by the trafficking of APP and its secretases, we and others hypothesized that endosomal trafficking defects are a causal mechanism of LOAD. Our goal is to dissect the mechanisms whereby genetic risk factors and neuronal aging alter endocytic trafficking to potentiate Aβ42 production. We analyze wild-type primary mouse cortical neurons matured or aged in culture, transduced with shRNA or overexpressing mutant cDNA, using a sensitive cell biological and neurobiological approach to determine APP and BACE1 trafficking alterations and their impact in the Aβ42 production. We have discovered that AD patients’ mutations affect Bin1 and CD2AP function increase beta-amyloid endocytic production, recapitulating the impact of Bin1 and CD2AP knockdown on BACE1 recycling and APP sorting to lysosomal degradation, suggesting their loss of function. Importantly, we discovered that neuronal aging alone potentiates APP clathrin and actin-mediated endocytosis, increasing intracellular Aβ42. Our results identify specific endocytic trafficking defects that the NRF2 pathway may overcome to prevent intracellular amyloid accumulation driven by genetic risk factors and aging, with the potential to delay late-onset Alzheimer’s disease.

Cláudia Guimas Almeida is Principal Investigator of the Neuronal Trafficking in Aging lab at CEDOC, the Chronic Research Center at NOVA Medical School (NMS) in Lisbon since 2013, supported by the Portuguese Science Foundation (FCT). Ph.D. in Neurosciences (2007) in the Gunnar Gouras lab (Weill Medical College of Cornell University; USA). Cláudia was an EMBO and a Marie Curie Post-doc fellow in Cell biology (2007-2012) at the Institut Curie (France) in Daniel Louvard lab. At CEDOC-NMS, Cláudia is co-coordinator of the Biomedical Research master program (NBR), the scientific Coordinator Microscopy facility, a member of NMS Scientific Council. Cláudia has 19 publications (>4000 citations) on intracellular trafficking mechanisms in healthy and Alzheimer’s disease cells. Her group discovered the cellular mechanisms whereby two top genetic risk factors, Bin1 and CD2AP, contribute to amyloid endocytic production in late-onset Alzheimer’s disease (EMBO reports 2017, JBC 2021), and whereby normal aging potentiates amyloid production, which may to aging-dependent synaptic decline (Journal of Cell Science 2021). Her group is investigating the mechanisms of synaptic decline in aging and late-onset Alzheimer’s disease to identify novel therapeutic targets to delay or treat Alzheimer’s disease.
Sorafenib downregulates nuclear factor E2-related factor 2 (Nrf2)-regulated thioredoxin 1 (Trx1) expression in liver cancer cells
Jordi Muntané
Department of Medical Physiology and Biophysics, University of Seville, Seville, Spain
E-mail: jmuntane-ibis@.es
Hepatocellular carcinoma (HCC) represents 80% of the primary hepatic neoplasms. It is the sixth most frequent neoplasm, the fourth cause of cancer-related death, and 7% of registered malignancies. Although immunotherapy and antiangiogenic combined treatment is emerging as first line therapy, Sorafenib is a useful treatment in the actual pipeline for patients in advanced stage of HCC. The administration of Sorafenib, and other tyrosine kinase inhibitors, is widely associated with mitochondrial dysfunction and the generation of reactive oxygen (ROS) and nitrogen (RNS) species. However, Sorafenib exerts a role as a free radical scavenger assessed by electron paramagnetic resonance, EPR. We also observed that Sorafenib downregulates nuclear factor E2-related factor 2 (Nrf2)-regulated thioredoxin 1 (Trx1) expression in liver cancer cells. In order to elucidate the function of Trx1 in our system, siRNA strategies and/or its overexpression showed that Trx1 induced activation of nitric oxide synthase (NOS) type 3 (NOS3) and S-nitrosation (SNO) of CD95 receptor leading to an increase of caspase-8 activity and cell proliferation, as well as reduction of caspase-3 activity in liver cancer cells. Sorafenib also transiently increased mRNA expression and activity of S-nitrosoglutathione reductase (GSNOR) in HepG2 cells. Different experimental models of hepatocarcinogenesis based on the subcutaneous implantation of HepG2 cells in nude mice, as well as the induction of HCC by diethylnitrosamine (DEN) confirmed the relevance of Trx1 downregulation during the proapoptotic and antiproliferative properties induced by Sorafenib. In conclusion, the induction of apoptosis and antiproliferative properties by Sorafenib were related to Trx1 downregulation that appeared to play a relevant role on SNO of NOS3 and CD95 in HepG2 cells. The transient increase of GSNOR might also participate in the deactivation of CD95-dependent proliferative signaling in liver cancer cells.

Jordi Muntané is Associate Professor in the Department of Medical Physiology and Biophysics, School of Medicine, University of Seville (Spain). The group is deciphering the molecular mechanism related to the antitumoral properties of tyrosine kinase inhibitors (TKIs) in liver cancer cells. In particular, the impact in mitochondrial dysfunction, oxidative and nitrosative stress and cell metabolism, endoplasmic reticulum stress, autophagy and apoptosis in the effectiveness of TKIs in liver cancer cells. The translational impact of the research involves the identification of circulating tumor cells (CTCs), extracellular vesicles (EVs) and miRNA and lncRNA signatures in blood from patients used as prognostic value of the disease and treatment response in patients with advanced HCC. In particular for the participation of our group in BenBedPhar network we will be focus on: 1) Molecular mechanism of Nrf2 regulation by TKIs. 2) Impact of NRf2-regulated genes in the antitumoral properties of TKIs and 3) Impact of drugs regulating Nrf2 in liver cancer cells.
- 15:30-16:00 Coffee break. Breakout rooms
- 16:00 - 17:30 SESION 3.Chair: Brigitte Winklhofer-Roob
NRF2 and dendritic cell tolerogenicity
Đorđe Miljković
Department of Immunology, IBISS, University of Belgrade, Serbia
Email: djordjem@ibiss.bg.ac.rs
Tolerogenic cell-based therapy is a promising approach for the treatment of uncurable autoimmune disease, such as multiple sclerosis and type 1 diabetes. Tolerogenic dendritic cells (DC) have been successfully applied in animal models of autoimmune diseases, and they are currently investigated in clinical trials. The field has been continuously expanded with investigation of novel compounds that can be used for induction of tolerogenic DC with superior functional properties. Accordingly, we have recently investigated tolerogenic effects of ethyl pyruvate and benfotiamine on DC. Both compounds have pronounced tolerogenic effects on DC. NRF2 activation has been identified as one of the mechanisms involved in their tolerogenic influence. Moreover, literature search shows that NRF2 signalling is highly relevant for tolerogenicity of DC. Thus, it is our opinion that potentiation of NRF2 in DC with specific NRF2 stimulators should be explored in details as a strategy for generation of next generation tolerogenic DC.

Đorđe Miljković is research professor and head of the Department of Immunology at the Institute for Biological Research “Siniša Stanković”, University of Belgrade. He studies cellular and molecular mechanisms involved in pathogenesis of autoimmune diseases. His current main research interests are: role of gut immune cells in autoimmunity, mechanisms of autoimmunity progression/regulation, cell-based therapy of autoimmunity (tolerogenic dendritic cells, regulatory T cells), modulation of autoimmune diseases by synthetic and natural compounds, mutual influence of autoimmunity and sepsis.
AMP(K)lifying Nrf2: The crosstalk between Nrf2 and the metabolic master hub AMPK
Elke H Heiss
Department of Pharmaceutical Sciences, University of Vienna, Vienna, Austria
Email: elke.heiss@univie.ac.at
The transcription factor Nrf2 (nuclear factor (erythroid-derived 2)-like 2) and the kinase AMPK (AMP-activated protein kinase) are involved in the adaptive homeostatic response to redox or energy stress. Despite accumulating evidence for positive cooperativity between both proteins, e.g. due to common activating stimuli or overlapping downstream effects, detailed information about post-translational modification of Nrf2 or about (epigenetic) control of Nrf2-dependent gene transcription by AMPK is relatively scarce or largely based on coincidental observations. Our previous studies showed that activated AMPK leads to phosphorylation of Nrf2 at three different serine residues in living cells, with no influence on half-life or nuclear translocation of Nrf2. However, the phosphorylation boosts or dampens expression of subsets of Nrf2 target genes. At the same time, AMPK did not significantly affect chromatin opening at examined Nrf2-dependent regulatory regions, but occurred to reduce expression of Bach 1 (BTB and CNC homology 1), a competitor of Nrf2 for ARE sites (with mainly repressor function). Hence, in AMPK-/- cells selected examined ARE sites are preferentially bound by Bach1 and not by Nrf2, which could provide an explanation for the observed reduced transactivation of some Nrf2 targets in the absence of AMPK activation. Currently, we dig deeper into the molecular interplay between AMPK, Nrf2 and Bach1 in order to better understand how the cellular energy state affects the Nrf2-dependent stress response, which could be of relevance for cellular stress resilience in states of overnutrition, caloric restriction or ageing.

Elke H. Heiss is Associate Professor of Pharmaceutical Biology at the Department of Pharmaceutical Sciences at the University of Vienna in Austria. She is highly interested in sigaling pathways involved in the cellular stress resistance and their rational modulation by small (natural) molecules. For the past years her main (cell-based/ in vitro) research activities have been focussed on (i) dissecting the crosstalk betweeen redox and energy stress signaling and on (ii) identification/characterization of (novel natural/ nature derived) small chemical entities with benefit for the cell`s capactity to efficiently cope with stress.
ulforaphane prevents age-associated cardiac, muscular, and skin dysfunction through Nrf2 signaling
Eugenia Carvalho
Center for Neuroscience and Cell Biology, University of Coimbra, Portugal
Email: ecarvalh@cnc.uc.pt
Age-associated mitochondrial dysfunction and oxidative damage are primary causes for multiple health problems including sarcopenia, cardiovascular disease (CVD) and skin aging, all related with inflammation. Though the role of Nrf2, a transcription factor that regulates cytoprotective gene expression, remains poorly defined, it has shown beneficial properties in both sarcopenia and CVD. Sulforaphane (SFN), a natural compound Nrf2-related activator of cytoprotective genes, provides protection in several disease states including CVD and is in various stages of clinical trials, from cancer prevention to reducing insulin resistance and more recently in skin aging. Our studies aimed to determine whether SFN could present age related loss of function in tissues such as, heart, skeletal muscle, and skin, in 2- and 22-month-old C57Bl6 mice, and whether these alterations may relate to changes in the microbiome. The results revealed a significant drop in Nrf2 activity and mitochondrial functions, together with a loss of skeletal muscle, cardiac and skin function in the old control mice compared to the younger age group. In the old mice, SFN restored Nrf2 activity, mitochondrial function, cardiac function, exercise capacity, glucose tolerance and the antioxidant capacities of the skin, together with a significant reduction in reactive oxygen species and matrix metalloproteinase 9 levels. These alterations were accompanied by the restauration of the gut microbiome, with enrichment of bacteria known to be associated with an improved intestinal barrier function and the production of anti-inflammatory compounds.

Dr. Carvalho studies the underlying mechanisms that are in the origin of insulin resistance and diabetes and related complications, across the lifespan. She has discovered that low insulin receptor substrate-1 protein levels in adipose tissue are predictors of pre-diabetes. She investigates specific biomarkers and pathways that are associated with insulin action and metabolic dysfunction in insulin sensitive tissues, from bench to bedside. In addition, she has been evaluating how specific markers are important in tissue regeneration under diabetes conditions. Recently, she has started to investigate the effect of nutraceuticals to improve insulin action and metabolism, including essential amino acids and sulforaphane, among other. In the context of early insulin resistance development, inflammation, and aging.
https://www.ncbi.nlm.nih.gov/myncbi/1N_kjxvZYzSAP/bibliography/public/
https://orcid.org/0000-0001-6264-3632
https://www.cnc.uc.pt/en/research-group/obesity-diabetes-and-complications
A brief overview of small molecule inhibitors of Keap1 and β-TrCP as Nrf2 inducers
Geoff Wells
UCL School of Pharmacy, 29/39 Brunswick Square, London, WC1N 1AX
Email: g.wells@ucl.ac.uk
The therapeutic potential of Nrf2-inducing molecules spans several disease states including chronic neurodegenerative diseases, inflammatory conditions and possible roles in cancer chemoprevention. Inhibitors of Keap1 and β-TrCP have the potential to inhibit Nrf2 ubiquitination and thereby increase its transcriptional activity. Ligands that bind to Keap1 in a reversible manner have been widely described, spanning a range of binding affinities and physicochemical properties. Conversely inhibitors of β-TrCP have not been widely described and the development of inhibitors of this target present some ongoing challenges. Recent progress with both targets and the potential for effective ligands to serve as chemical probes for each target will be discussed.
Geoff Wells is an Associate Professor at UCL School of Pharmacy. His research interests are directed towards the discovery of agents for the prevention and treatment of cancer and other life threatening diseases. His research work has focused on the design and synthesis of compound classes that affect redox homeostasis, interact with DNA in a sequence selective manner and that have selective cytotoxicity profiles. His work to date has resulted in the publication of several patents for agents under preclinical investigation. His current interests include the rational design of agents that interact with molecular targets of relevance to the chemoprevention of cancer such as the Keap1-Nrf2 system involved in the regulation of antioxidant response element genes.
The expression pattern of inflammation and redox genes in the blood of mild AD patients – focus on the molecular signatures of NFkB and NRF2
Gina Manda
Radiobiology Laboratory, “Victor Babes” National Institute of Pathology, Romania
Email: gina.manda@gmail.com
Besides the association of the Alzheimer’s disease (AD) with profound alterations of the central nervous system, this disease seems to have an echo in blood which might provide valuable peripheral biomarkers for improved diagnosis, prognosis and drug response monitoring. In this context, we performed a targeted transcriptomic study on 38 mild AD patients and 38 matched controls for evaluating the expression levels of 136 inflammation and 84 redox genes in whole blood. Patients were diagnosed as mild AD based on altered levels of total TAU, phospho-TAU and Abeta(1–42) in the cerebrospinal fluid, and Abeta(1–40), Abeta(1–42) and total TAU levels in plasma. We evidenced 48 inflammation and 34 redox genes differentially expressed in the blood of AD patients vs controls (FC>1.5, p<0.01), out of which 22 pro-inflammatory and 12 redox genes exhibited a higher level of up-regulation (FC>2, p<0.001). Using the receiver operating characteristic analysis, we finally selected nine inflammation and seven redox candidate genes that discriminated well between AD patients and controls (area under the curve>0.9). The correlations between the abnormal blood levels of inflammation and redox transcripts in mild AD suggest that RELA might regulate the expression of several redox genes, as for example DUOX1 and GSR. The gene expression pattern revealed that the transcription factors NFκB and NRF2, master regulators of inflammation and redox homeostasis, were significantly disturbed in the blood of AD patients. Concluding, the selected inflammation and redox genes might be useful biomarkers for monitoring antioxidant and anti-inflammatory co-therapies in mild AD. These preliminary results have to be further validated in a larger independent cohort, including investigations at protein level

Gina Manda is the head of the Radiobiology laboratory at “Victor Babes” National Institute of Pathology, Bucharest, Romania. She is currently studying the molecular mechanisms underlying: i) low-grade inflammation and disturbed redox signaling in chronic diseases (Alzheimer’s disease), and ii) the responses of normal and tumor cells elicited by radiation exposure in experimental settings relevant for anti-cancer therapies (radio- or photodynamic therapy) or for space medicine. One of the main interests is to develop at preclinical level therapies that target NRF2, in order to: i) increase the efficacy of anti-cancer therapies using targeted NRF2 inhibitors; ii) counteract using NRF2 activators the deleterious effects of galactic cosmic radiation on astronauts during long-term travel in the deep space, an issue that is highly relevant in this era of intensive preparations for space exploration.
Therapeutic effects of HYCOs, hybrid molecules that activate Nrf2/HO-1 and simultaneously release carbon monoxide
Roberto Motterlini and Roberta Foresti
Faculty of Health, University Paris-Est Créteil, INSERM, Créteil, France
Email: roberto.motterlini@insrrm.fr
Carbon monoxide (CO) produced by heme oxygenase-1 (HO-1) or delivered by CO-releasing molecules (CO-RMs) exerts anti-inflammatory action, a feature also exhibited by the nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of the stress response. We have recently developed new hybrid molecules (HYCOs) consisting of CO-RMs conjugated to fumaric esters known to activate Nrf2/HO-1. Here we evaluated the biological activities of manganese (Mn) and ruthenium (Ru)-based HYCOs in human monocytes and keratinocytes in vitro as well as in vivo models of inflammation. The effects of HYCOs were compared to: a) dimethyl fumarate (DMF), a known fumaric ester used in the clinic; b) a CO-RM alone; or c) the combination of the two compounds. Mn-HYCOs donated CO and up-regulated Nrf2/HO-1 in vitro more efficiently than Ru-HYCOs. However, irrespective of the metal, a strong reduction in anti-inflammatory markers in monocytes stimulated by LPS was observed with specific HYCOs. This effect was not observed with DMF, CO-RM alone or the combination of the two, indicating the enhanced potency of HYCOs compared to the separate entities. Selected HYCOs given orally to mice accelerated skin wound closure, reduced psoriasis-mediated inflammation and disease symptoms equalling or surpassing the effect of DMF, and ameliorated motor dysfunction in a mouse model of multiple sclerosis. Thus, HYCOs have potent anti-inflammatory activities that are recapitulated in disease models in which inflammation is a prominent component. Prolonged daily administration of HYCOs (up to 40 days) is well tolerated in animals. Our results clearly confirm that HYCOs possess a dual mode of action highlighting the notion that simultaneous Nrf2 targeting and CO delivery could be a relevant application to combat inflammation.

Roberto Motterlini is Director of Research (DR1) at INSERM U955 within the Faculty of Health, University of Paris Est, France. He has a long-standing interest in the regulation, activity and biological significance of heme oxygenase-1 (HO-1), a ubiquitous defensive protein that degrades heme to carbon monoxide (CO) and biliverdin. His studies focused on the role of Nrf2 as a transcription factor in controlling HO-1 gene expression and uncovered the vasodilatory, anti-ischemic and anti-inflammatory properties of CO. His research led to the development of CO-releasing molecules (CO-RMs), small active compounds that deliver controlled amounts of CO in vivo and have been shown to exert important pharmacological actions to counteract vascular, inflammatory and metabolic disorders. More recently, Dr. Motterlini’s group has characterized a new class of hybrid compounds, termed HYCOs, which have the ability to activate Nrf2 and simultaneously release CO. These hybrid molecules with dual function have been demonstrated to exert therapeutic effects in inflammatory models of disease such as skin wound, psoriasis and multiple sclerosis.
- 17:30-18:00. Breakout rooms
January 11
- 9:30-11:15 SESION 4. Chair: Guy Cohen
Lack of transcriptionally active Nrf2 mitigates colon dysfunction in female mice – the role of estrogen receptors.
Aleksandra Piechota-Polanczyk
Department of Medical Biotechnology, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, Krakow, Poland
Email: aleksnadra.piechota-polanczyk@uj.edu.pl
The nuclear factor-erythroid-2-related factor 2 (Nrf2) is a key transcription factor regulating the cellular reduction-oxidation homeostasis and influencing the expression of numerous signaling pathways. We observed that Nrf2 transcriptional knockout (tKO) mice have inflammatory bowel diseases-like changes in the colon at basal condition. Additionally, these changes appear rather in females than males and lapse with age. However, those potentially harmful features do not influence mouse development and disease manifestation. Therefore, we aimed to verify how the lack of transcriptional activity of Nrf2 influences functionality of the intestines in young (3 m.o.) and older (6 m.o.) female mice with the functional Nrf2 (WT) or with the transcriptionally inactive form of Nrf2 (tKO). Moreover, to verify the role of estrogens in Nrf2 tKO females, some mice had implemented 17beta-estradiol-releasing (0.7- 1.3 µg/day) or placebo implants (n=6 per group and test). Mice were subjected to functional tests of gastrointestinal track activity which included whole gastrointestinal transit, castor-oil induced diarrhea and colonic bead expulsion. After testing, mice were euthanized, venous blood was collected, and the intestines were dissected. The total macroscopic damage score was calculated for each animal and intestinal samples were preserved for histological staining and biochemical analysis of inflammation and estrogen receptors localization. The results indicated an age-dependent alterations in Nrf2 tKO females which included changed gastrointestinal tract function, microscopic alterations in the proximal colon, higher expression of estrogen receptor alpha as well as lower level of GPR30 receptors in the intestines. Additionally, treatment with 17beta estradiol influenced gastrointestinal tract functionality and estrogen receptors localization in Nrf2 tKO mice. Therefore, lack of transcriptionally active Nrf2 may influence function of gastrointestinal tracks in female mice and the estrogen receptor signaling may be implicated in those changes. (Study supported by the Sonata 14 program of the National Science Centre 2018/31/D/NZ4/00077 to APP).

Aleksandra Piechota-Polanczyk is associate professor at the Department of Medical Biotechnology, at the Jagiellonian University in Krakow, Poland. Her research interests focus on finding new anti-oxidative and anti-inflammatory proteins that could be potential markers and/or targets in treatment of inflammatory bowel diseases and cardiovascular diseases. Currently she is a PI of the project “Fibrosis or senescence – why lack of transcriptionally active Nrf2 protects against colon dysfunction”, where she wants to evaluate the role of Nrf2 in the causes of fibrosis and cellular senescence in vivo and in vitro.
The anti-inflammatory role of Nrf2 activation
Albena T. Dinkova-Kostova
Division of Cellular Medicine, University of Dundee School of Medicine, United Kingdom
Email: a.dinkovakostova@dundee.ac.uk
Transcription factor Nrf2 and its main negative regulator Keap1 are at the interface of redox and intermediary metabolism. Nrf2 activation by small molecules that target Keap1 (termed inducers) provides protection via the transcriptional regulation of large networks of cytoprotective proteins. Additionally, pharmacologic Nrf2 activation linearly correlates with inhibition of pro-inflammatory responses. Studies in cellular and mouse models, as well as in human subjects consistently show the anti-inflammatory role of Nrf2 activation, although the underlying mechanisms are complex and incompletely understood. Recent high-resolution quantitative proteomics coupled with metabolomic analyses of bone marrow-derived macrophage (BMDM) cells from wild-type-, Nrf2-knockout and Keap1-knockdown mice showed that Nrf2 disruption significantly affected the proteome and metabolome of unstimulated and lipopolysaccharide (LPS)-stimulated BMDM cells, with alterations in redox, carbohydrate and lipid metabolism, and innate immunity. Interestingly, LPS stimulation caused a switch in mitochondrial morphology, from tubular to fused, which was enhanced by Keap1 knockdown and suppressed by Nrf2 disruption. The Nrf2 activator, 4-octyl itaconate (4-OI) remodelled the inflammatory macrophage proteome, increasing redox and suppressing the type I interferon (IFN) response in Nrf2-dependent manner. Similarly, pharmacologic or genetic Nrf2 activation inhibited transcription of IFN-β and its downstream effector IFIT2 during LPS stimulation. Overall, these data suggest that Nrf2 activation facilitates metabolic reprogramming and mitochondrial adaptation, and finetunes the innate immune response in macrophages.

Albena Dinkova-Kostova is Professor of Chemical Biology at the University of Dundee School of Medicine. She graduated in Biochemistry and Microbiology from Sofia University (Bulgaria) and obtained her PhD degree in Biochemistry and Biophysics from Washington State University (USA). She subsequently trained in Pharmacology at Johns Hopkins University School of Medicine (USA), where she continues to hold an Adjunct Professor position. She joined the University of Dundee in 2007 as a Research Councils UK Academic Fellow. In her research, at the interface of Chemical Biology and Medicine, she is committed to understanding the role of Keap1 and Nrf2 in mediating the cellular and organismal responses to oxidative, inflammatory, and metabolic stress, and is working towards development of pharmacological Nrf2 activators for protection against chronic disease. She was named among the top influential academics in Clarivate’s Highly Cited Researchers 2019, 2020, and 2021 lists.
Translational implications of Nrf2 signaling in the thyroid
Gerasimos P. Sykiotis
Service of Endocrinology, Diabetology and Metabolism, Lausanne University Hospital and University of Lausanne, Switzerland
Email: gerasimos.sykiotis@chuv.ch
The synthesis of thyroid hormones depends on the intake of miconutrients including iodine and selenium, and it entails the active production of reactive oxygen species (ROS) in thyroid follicular cells to oxidize iodine and iodinate thyroglobulin, the thyroid hormone precursor. At the same time, like all other tissues, the thyroid gland must also defend itself against oxidative stress, for which it relies on antioxidant defense systems to maintain its own homeostasis in the face of continuous self-exposure to ROS. Over the last few years, my group has identified multiple links between the Keap1/Nrf2 antioxidant response pathway and thyroid physiology, as well as various thyroid pathologies, including autoimmunity, goiter, hypothyroidism, hyperthyroidism, and cancer. This presentation will summarize the main results of these studies and will highlight future perspectives, with a focus on translational implications (i) for the pathogenesis and treatment of thyroid diseases; (ii) for basic research studies using animals with genetically or pharmacologically modified Nrf2 activity; and (iii) for the use of Nrf2-modulating compounds in clinical studies..

Gerasimos (Gerry) Sykiotis is associate professor at the Faculty of Biology and Medicine of the University of Lausanne and senior staff physician in the Service of Endocrinology, Diabetology and Metabolism at Lausanne University Hospital. He is a physician-scientist specialized in clinical and basic endocrinology with a particular focus on thyroid physiology and thyroid diseases, including hyperthyroidism, hypothyroidism and thyroid cancer. Since 2015, he is responsible for the thyroid endocrine clinic at Lausanne University Hospital. His clinical research focuses on the needs of patients with thyroid diseases, including quality of life among thyroid cancer patients and survivors. His basic research, funded primarily by the Swiss National Science Foundation, focuses on the roles of cellular homeostatic systems in thyroid physiology and pathophysiology.
Nrf2 in stress, aging and age-related diseases
Ioannis Trougakos
Department of Cell Biology and Biophysics, Faculty of Biology, National and Kapodistrian University of Athens, Athens, 157 84, Greece
Email: itrougakos@biol.uoa.gr
Viability of metazoans largely depends on their ability to regulate metabolic processes in order to produce energetic molecules as well as on their capacity to mount anti-stress responses. These processes are regulated in real-time by a network of sensors (mostly transcription factors) which monitor organismal physicochemical parameters and constantly trigger genomic responses aiming to restore optimal (evolutionary set) values and normal cellular functionality. At the whole organism level, these responses require complex co-regulation and wiring of cell-autonomous and non-autonomous mechanisms; which however, decline during aging leading to increased morbidity and mortality. The network of cellular sensors comprises numerous short-lived proteins, including nuclear factor erythroid 2 like 2 (NFE2L2/Nrf2) which reportedly modulates cell responses against (among others) oxidative/xenobiotic and proteome damage. We will discuss short- and long-term effects of Nrf2 activation and also our findings showing that persistent stress signaling via sustained Nrf2 activation triggers an adaptive metabolic response which reallocates resources from growth and longevity to somatic preservation and stress tolerance. Moreover, our efforts to therapeutically target the Nrf2 pathway for anti-aging purposes, including a detailed understanding of the correct time- (when), dose- (how much) or tissue- (where) targeted interventions will be presented.

Ioannis Trougakos obtained his Ph.D. in Cellular-Developmental Biology from the University of Athens (UoA), Greece. He has worked as Research Scientist at the European Molecular Biology Laboratory, Germany; at the Centro De Biologia Molecular “Severo Ochoa”, Spain and at the National Hellenic Research Foundation, Athens, Greece; he was also research visitor at the Netherlands Cancer Institute. Currently he serves as Professor and Director of the “Cell Biology” lab at the Faculty of Biology, UoA; he directs three MSc UoA courses, he is vice Chairman of the Faculty of Nutrition and Dietetics, UoA, a member of the Coordination Board of the UoA Centre of Excellence on “Bioactive Natural Products” and also adjunct Professor of “Systems Biology of Ageing and Cancer”, at the European University Cyprus, Cyprus. Prof. Trougakos has received fellowships from the EU and the Hellenic State; has participated in many international courses, has been honored with various awards and he was an invited lecturer in international conferences and Universities; he was also invited Reviewer for the 2019 World Cancer Report. His scientific-research interests are focused on the understanding of the molecular-cellular basis of ageing and age-related diseases. He has published >165 Publications/Chapters in Peer Reviewed International Scientific Journals or Books. His group is funded by National and International grants, by foundations, as well as by contractual activities with the industry.
Quercetin analogues and derivatives for pharmacological regulation of NRF2
Kateřina Valentová
Institute of Microbiology of the Czech Academy of Sciences, Prague, Czechia
Email: kata.valentova@email.cz
Various flavonoids, such as luteolin, apigenin, quercetin, myricetin, rutin, naringenin, epicatechin, and genistein as well as (2,3-dehydro)flavonolignans (2,3-dehydrosilydianin) are known to activate the Nrf2/ARE signaling pathway in both normal and cancer cells. Nrf2 regulates the expression of about 250 genes encoding a network of mostly cytoprotective enzymes involved in NADPH-, glutathione- and thioredoxin-mediated responses, inhibition of inflammation, induction of autophagy genes, and so on. Through this transcriptional network, Nrf2 coordinates numerous responses to various forms of stress to maintain a stable internal environment. The most important mechanism of Nrf2 regulation is the control of protein stability by KEAP1. Pharmacological research on Nrf2 targeting KEAP1 is well advanced in preclinical models of various non-communicable diseases and is now beginning to evolve to the level of clinical practice. Activation of Nrf2 pathway (and probably not their direct antioxidant activity, as usually considered) also plays a role in flavonoid-mediated protection by inducing various cytoprotective genes. However, their action is strongly influenced by their metabolization and depends on concentrations (hormetic effect, antioxidant/prooxidant activity). For BenBedPharm, we will synthesize a small library of natural flavonoids and flavonolignans and their derivatives with Nrf2-modulating potential for SAR studies.

Kateřina Valentová is an Associate Professor of Medicinal Chemistry and Biochemistry from the Faculty of Medicine and Dentistry of Palacký University in Olomouc and Head of the Laboratory of Biotransformation at the Institute of Microbiology of the Czech Academy of Sciences in Prague. Her main professional interests include biologically active natural products, mainly (poly)phenols, their bioavailability, metabolism and biotransformation, but also their biological activity including the effect on signaling pathways. Her team consists of synthetic chemists experienced in modifications of flavonoid scaffold.
Transcriptional Activation of NRF2 Defense Genes is Attenuated in Vascular Cells under Physiological Normoxia: Consequences for Reperfusion Injury
Giovanni E. Mann
British Heart Foundation Centre of Research Excellence, Faculty of Life Sciences & Medicine, King’s College London, London SE1 9NH, U.K.
Email: giovanni.mann@kcl.ac.uk
We previously extablished in a rodent model of ischemic stroke that activation of NRF2 defenses by pretreatment with sulforaphane (SFN) affords protection against neurovascular and neurological deficits. To further investigate the molecular mechanisms, we further investigated SFN-mediated protection in mouse brain microvascular endothelial cells (bEnd.3) adapted long-term to hyperoxic (18 kPa), normoxic (5 kPa) or hypoxic (1 kPa) O2 levels. Using an O2-sensitive phosphorescent nanoparticle probe, we measured an intracellular O2 level of 3.4 ± 0.1 kPa in bEnd3 cells cultured under 5 kPa O2. Induction of HO-1 and GCLM by SFN (2.5 µM) was significantly attenuated in cells under 5 kPa O2, despite nuclear accumulation of Nrf2. To simulate ischemic stroke, cells were adapted to 18 or 5 kPa O2 and subjected to hypoxia (1 kPa O2, 1h) and reoxygenation. In cells adapted to 18 kPa O2, reoxygenation induced free radical generation was abrogated by PEG-SOD and significantly attenuated by pretreatment with SFN (2.5 M). Silencing NRF2 abrogated HO-1 and NQO1 induction and led to a significant increase in reoxygenation induced free radical generation. Notably, reoxygenation induced oxidative stress, assayed using the luminescence probe L-012 and fluorescence probes MitoSOXTM Red and FeRhoNoxTM-1, was diminished in cells cultured under 5 kPa O2, indicating an altered redox phenotype in brain microvascular cells adapted to physiological normoxia. As redox and other intracellular signaling pathways are critically affected by O2, the development of high throughput therapies targeting KEAP1-NRF2 in the treatment of reperfusion injury in stroke, coronary and renal disease will require the design of in vitro studies to recapitulate the redox phenotype of cells in vivo (see Keeley & Mann, Physiol. Rev. 2019;99(1):161-2340).

Giovanni E. Mann is Professor of Vascular Physiology in the British Heart Foundation Centre of Research Excellence, Faculty of Life Sciences & Medicine, King’s College London, UK, President of SFRRi and Editor of FRBM Reviews and Special Issues. His research group is investigating Nrf2 transcriptional activation of antioxidant defense genes in vascular endothelial and smooth muscle cells exposed to oxidative stress in diseases such as gestational diabetes and ischemic stroke. He demonstrated the critical importance of conducting cell culture under oxygen levels relevant to specific tissues and organs. His research encourages a paradigm shift in the field, enabling researchers and Pharma to improve translation of molecular findings in vitro to disease pathology and high-throughput screening of NRF2 therapeutics for treatment coronary heart disease, ischemic stroke and wider field of vascular biology.
CO-mediated cytoprotection is dependent on cell metabolism modulation – implication on neuronal differentiation, anti-inflammation role and prevention of cell death
Helena L.A. Vieira
UCIBIO, Applied Molecular Biosciences Unit, Department of Chemistry, NOVA School of Science and Technology, Universidade Nova de Lisboa, Portugal
Carbon monoxide (CO) is a gasotransmitter endogenously produced by the activity of heme oxygenase, which is a stress-response enzyme and is under the control of Nrf2. Endogenous CO or low concentrations of exogenous CO have been described to present several cytoprotective functions: anti-apoptosis, anti-inflammatory, vasomodulation, maintenance of homeostasis, stimulation of preconditioning and modulation of cell differentiation. Herein it is demonstrated and discussed how CO regulates cell metabolism and how it is involved in the distinct cytoprotective roles of CO. Using different brain cell models (neurons, astrocytes and microglia) we have demonstrated that CO promotes mitochondrial ROS generation, stimulation of mitochondrial biogenesis and increased oxidative phosphorylation. Likewise, CO negatively regulates glycolysis and improves pentose phosphate pathway. These alterations in cell metabolism are implicated in: (i) prevention of astrocytic cell death; (ii) improvement of neuronal differentiation and (iii) anti-neuroinflammatory effect in microglia.
Publications related to the talk
• Dias-Pedroso D., Ramalho J.S., Sardão V.A., Jones J.G., Romão C.C., Oliveira P.L. and Vieira H.L.A, Carbon monoxide-Neuroglobin axis targeting metabolism against inflammation in BV-2 microglial cells, Molecular Neurobiology, 2021, doi: 10.1007/s12035-021-02630-4
• Almeida A.S., Soares N.L., Sequeira CO, Pereira S.A., Sonnewald U., Vieira H.L.A. Improvement of neuronal differentiation by carbon monoxide: role of pentose phosphate pathway. Redox Biol. 2018 Jul 17:338-347. doi: 10.1016/j.redox.2018.05.004
• Almeida A.S., Sonnewald U., Alves P.M. and Vieira H.L.A., “Carbon monoxide improves neuronal differentiation and yield by increasing the functioning and number of mitochondria”, Journal of Neurochemistry, 2016, Apr 29. doi: 10.1111/jnc.13653
• Almeida A.S., Queiroga C.S.F., Sousa M.F., Alves P.M. and Vieira H.L.A., Carbon monoxide modulates apoptosis by reinforcing oxidative metabolism in astrocytes: role of Bcl-2, Journal of Biological Chemistry 2012, 287: 10761-70
- 11:15-11:30 Coffee break. Breakout rooms
- 11:30-13:00 SESION 5. Chair: Brigitta Buttari
Mild hypothermia alleviates reductive stress, a root cause of ischemia reperfusion injury
Kattri-Liis Eskla
Department of Physiology, Institute of Biomedicine and Translational Medicine, University of Tartu, Tartu, Estonia
Email: kattri-liis.eskla@ut.ee
Over the past 15 years, therapeutic hypothermia has proven to have potential to be one of the most attractive therapies for ischemic stroke, however, uncertainty around why and how hypothermia (32°C) provides protection is still a challenge.
Recent evidence suggests that markedly increased reducing power (i.e. the accumulation of reducing equivalents such as succinate) is the root cause of ischemia reperfusion injury. Our results demonstrate that hypothermia at least partly mitigates the dangers posed by reductive equivalents. During ischemic stroke, for example, ATP synthesis is inhibited. The most well known therapeutic mechanism of hypothermia is the preservation of ATP. In line with these observations, we found that hypoxia reduces ATP/ADP ratio at 37°C while mild hypothermia (32°C) increases ATP/ADP ratio in both normoxic and hypoxic cells. Another mechanism for mitigating the dangers posed by excess reducing equivalents is the channeling of acetyl-CoA into lipid droplets, which are chemically more stable depos of reductive power. As expected, lipid content was increased by hypoxia. However, mild hypothermia prevented hypoxia-dependent increase in lipid content. Furthermore, hypoxia-dependent increase in PPARg gene expression (fatty acid uptake) was abolished by hypothermia. Another mechanism to counteract the buildup of reducing equivalents is the antioxidant system that seeks to neutralize reactive oxygen species. In a recent study our group has demonstrated for the first time that mild hypothermia activates Nrf2, a major regulator of antioxidant gene transcription, in normoxic cells and provides protection from oxidative stress presumably by orchestrating adaptive responses to redox stress.
Numerous studies suggest that reduced blood flow and oxygen deprivation result in the decoupling of citric acid cycle (CAC) and electron transport chain (ETC) as evidenced by ischemia reperfusion injury. We have recently developed a unique cellular respiration monitoring system to determine O2 and CO2 fluxes in intact cells in real time. It allows us to measure the production (by CAC) and utilization (by ETC) of reducing power. Mild hypothermia reduced O2 and CO2 fluxes approximately 25%. In addition, hypothermia introduced during anoxia enhanced the recovery of respiration.
This study addresses an important question on why hypothermia is effective in reducing hypoxic tissue damage. It suggests that hypothermia alleviates reductive stress, a conceptually novel and largely overlooked phenomenon at the root of ischemia reperfusion injury.
Kattri-Liis Eskla is research fellow in Physiology at the Department of Physiology, Institute of Biomedicine and Translational Medicine, University of Tartu, Estonia. Her main research focus is understanding the role of hypoxia and hypothermia in the control of metabolism by using genetic models, biochemistry, molecular biology, measurement of O2 and CO2 in response to different nutrients and oxygen concentration, and state of the art metabolomics and tracing techniques.
Role of poly(ADP-ribosyl)ation in oxidative stress-induced cell death and inflammatory signaling
László Virág
Department of Medical Chemistry, Faculty of Medicine, University of Debrecen, Hungary
Email: lvirag@med.unideb.hu
Poly(ADP-ribose) polymerase (PARP) enzymes cleave NAD+ substrate into nicotinamide and ADP-ribose and attach the latter covalently to target proteins. Some enzymes of the 17 member PARP family can also polymerize ADP-ribose units onto protein targets resulting in their poly(ADP-ribosyl)ation (PARylation). Mono and poly(ADP-ribosyl)ation is involved in the regulation of various cellular processes including DNA repair, DNA replication, gene transcription and metabolism. In excessive DNA damage scenarios PARP1 activation can also cause cell death which is now recognized as a novel cell death entity called parthanatos. PARylation is a drug target in BRCA1/2 mutant cancers and preclinical data also suggest that PARP inhibition may provide therepeutic benefit in severe tissue injuries (e.g. in stroke and ischemia-reperfusion injury of the heart or intestines) and in various forms of inflammation. The underlying mechanism of the latter likely involves direct interaction of PARP1 with NFκB and AP-1 transcription factors.
Since macrophages (MΦ) are resistant to oxidative stress we set out to investigate the mechanism by which inflammatory (M1) MΦs protect themselves from oxidative stress. We found that selfprotection involves downregulation of PARP1 gene expression, upregulation of the expression of antioxidant enzymes and reprogramming cell metabolism. We are also investigating the mechanism by which PARylation regulates phenotypic changes of MΦs (e.g. shifts to M2 phenotype) that lead to the cancer promoting effects of MΦs. Moreover, we are developing several high-throughput screening (HTS) and High-Content Screening (HCS) applications to study cell fates in various cell biology models (e.g. cell death, antibody-dependent cell mediated cytotoxicity (ADCC), cell migration, autophagy) and carry out repurposing drug library screens in these applications. One of our goals in the BenBedPhar project is to collaborate with partners on revealing potential overlaps between Nrf2 and PARylation signaling and to participate in HTS/HCS-based identification of novel Nrf2 modulator drugs.

László Virág is full professor at and head of the Department of Medical Chemistry, Faculty of Medicine, University of Debrecen, Hungary. He studies the molecular mechanisms involved in oxidative DNA damage signaling with special regard to the role of protein PARylation. In recent years his research focused on the following lines of activity:
i) how do macrophages protect themselves from PARylation-dependent cell death (parthanatos);
ii) how PARylation regulates macrophage polarization in cancer;
Targeting the BACH1-NRF2 axis
Laureano de la Vega
Division of Cellular Medicine, University of Dundee, UK.
Email: l.delavega@dundee.ac.uk
NRF2 is a transcription factor that controls a wide variety of genes encoding for antioxidant, detoxification and anti-inflammatory proteins. Although the main negative regulator of the NRF2 pathway is KEAP1, there are additional factors that also negatively regulate the pathway. One of those factors is BACH1 (broad complex, tramtrack and bric à brac and cap’n’collar homology 1), a transcription factor that competes with NRF2 for its binding to the promoter of a subset of NRF2 target genes, being the potent antioxidant and anti-inflammatory enzyme HMOX1 the best characterised1. While KEAP1 inhibitors induce the expression of numerous cytoprotective genes, BACH1 inhibition will activate only a few, although they are extremely potent at inducing HMOX1. Additionally, BACH1 also activates genes involved in cancer metastasis (in a NRF2-independent manner)2. Based on this, BACH1 is a potential target against a variety of conditions linked to oxidative stress and inflammation, and also against cancer metastasis. Despite their therapeutic potential, only a few BACH1 inhibitors have been identified so far, and none has entered the clinical scenario yet.
In this talk I will provide new data comparing the use of HMOX1 as a reporter for NRF2 activation or as a reporter for BACH1 inhibition. I will explain the cellular model we use to identify BACH1 inhibitors and finally, I will present some recent data on the characterisation of novel potent dual KEAP1/BACH1 inhibitors and some of their potential use.
1. J. Sun et al., ‘Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene’, EMBO J., vol. 21, no. 19, pp. 5216–5224, Oct. 2002.
2. L. Lignitto et al., ‘Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1’, Cell, vol. 178, no. 2, pp. 316-329.e18, Jul. 2019
NRF2 and ROS in Cancer
Lidija Milkovic
Laboratory for Oxidative Stress, Division of Molecular Medicine, Rudjer Boskovic Institute, Zagreb, Croatia
Email: lidija.milkovic@irb.hr
Cancer remains a puzzle and a global burden with no general picture fitting due to cancer-known heterogeneity between cancers of different origins and within the same cancer as well. Numerous factors, including reactive oxygen species (ROS), are contributing to the development and the progression of cancer. ROS has a dual role in cancer. They can lead to genetic mutations, thus supporting cancer development, but they can also lead these transformed cells to apoptosis, thus inducing cancer growth arrest. Additionally, ROS together with the thioredoxin, peroxiredoxin, and glutathione systems, are vital in redox signaling, an important “cellular messaging system”. The nuclear factor erythroid 2-related factor 2 (NRF2), the major antioxidative transcription factor, controls the expression of numerous genes involved in redox homeostasis and regulates the levels of ROS, thus affecting redox signaling. NRF2 is known for its dual role in cancer as well, by reducing the ROS levels, NRF2 protects from cancer development, a feature often exploited by cancer cells thus governing their therapy resistance. Therefore, investigating the role of ROS on one side and NRF2 is essential in understanding what are the specific switches and in what situations they occur in cancer, elucidating the underlying mechanism(s), thus opening new possibilities in cancer treatment, preventing its development and progression to metastasis formation.
Lidija Milkovic is a Research Associate in the Laboratory for Oxidative Stress, Division of Molecular Medicine at the Rudjer Boskovic Institute in Zagreb, Croatia. She studies the role of oxidative stress, and concurrent antioxidative mechanisms, in diverse (patho)physiological processes focusing on cancer. In the last years, she was involved in a project investigating the “the driver mechanism” of cellular metabolic reprogramming through epigenetically modulated NRF2 activity in several cancer cell lines of different origins and genetic backgrounds, and its significance for patients with head and neck tumors. More recently, she works on a project of Dr. Ana Cipak Gasparovic (MC member of this COST Action) investigating the role of peroxiporins in the regulation of the cellular antioxidative system. She is also interested in understanding the intertwined regulation of antioxidative mechanisms, mainly of the NRF2 signaling, metabolic reprogramming, and the involvement of reactive oxygen species (ROS) in cancer. .
Implication of Nrf2/heme oxygenase-1 pathway in articular and skin diseases
M Carmen Montesinos y M Luisa Ferrándiz
Department of Pharmacology, Pharmacy School, University of Valencia, Spain
Email: monmez@uv.es, luisa.ferrandiz@uv.es
Autoimmune and chronic inflammatory diseases have a high prevalence and a negative impact on the quality of life of patients. Activation of the transcription factor Nrf2 results in the synthesis of heme oxygenase-1 (HO-1), leading to the formation of bioactive metabolites. We have demonstrated that Nrf2 and HO-1 can confer protection against oxidative stress and inflammatory responses in joint tissues. Thus, Nrf2 deficiency accelerated the incidence of arthritis, and animals showed a widespread disease, using the transfer of serum from arthritic K/BxN transgenic mice to Nrf2−/− mice. In addition, ovariectomized Nrf2−/− mice exhibit a loss in bone mineral density, accompanied by increased osteoblastic and osteoclastic markers, indicating that this transcription factor is indispensable for normal bone microarchitecture. Since HO-1 has shown anti-inflammatory effects in different systems, we investigated its influence on the acute inflammatory response to zymosan in the air pouch model in mice with myeloid-restricted deletion of HO-1 (HO-1M-KO), as well as on different models of arthritis (adjuvant-induced in rats, and collagen-induced or K/BxN serum transfer in mice) with various strategies: therapeutic administration of HO-1 inducers or inhibitors; and used of genetic modified mice (HO-1+/+, HO-1+/− and HO-1−/−). We have currently established different in vitro and in vivo protocols to study chronic inflammatory skin conditions, such as psoriasis, in which Nrf2 has shown to be protective. Further studies are necessary to identify improved strategies to regulate Nrf2 and HO-1 activation in order to enable the development of drugs with therapeutic applications in joint and skin diseases.

Mª Luisa Ferrándiz Manglano is full professor of Pharmacology at the University of Valencia. Her main lines of research include: anti-inflammatory and immunomodulatory activity of new compounds of natural and synthetic origin; experimental models of acute and chronic inflammation; implication of the Nrf2/heme oxygenase-1 pathway in the development and resolution of chronic inflammatory processes. In recent years, her research focuses on the development of animal models of arthritis and experimental osteoarthritis, studying the mechanisms involved in these chronic joint pathologies and evaluating new targets and therapeutic strategies.
Mª Carmen Montesinos is tenured professor of Pharmacology at the University of Valencia. Her main lines of research are related to the physiological effects of the activation of adenosine receptors and their involvement in promotion of wound healing and in the anti-inflammatory effect of anti-rheumatic drugs. Currently, her research focuses on the study of the pathological processes involved in inflammatory skin diseases in order to improve current therapeutic options.
Mitochondrial metabolism and endogenous antioxidants under NRF2 activation in the mechanism of neuroprotection in neurodegenerative disorders and epilepsy
Andrey Y. Abramov
Department of Clinical and Movement Neurosciences, UCL Queens Square Institute of Neurology, Queen Square, London, UK
Email: a.abramov@ucl.ac.uk
Most of the neurodegenerative disorders and some of neurological conditions such a epilepsy characterised by mitochondrial disfunction which results in energy deprivation and oxidative stress and neuronal loss. Nrf2 controls major endogenous antioxidant pathways and also support mitochondrial metabolism by substrates. We have found that pharmacological activation of Nrf2 restore energy metabolism and increase the level of GSH in the familial forms of Parkinson’s disease (PINK1 and SNCA triplications) and protect neurons against cell death. In epilepsy, KEAP1 inhibition (activation of Nrf2) is neuroprotective and suppresses the development of seizures. However, the most effective treatment of the epilepsy was the combination of the Nrf2 activation (pharmacological Keap1 inhibition) and inhibitor of NADPH oxidase. Importantly, this combination completely restore altered mitochondrial membrane potential, decrease seizure-induced ROS production and prevented the development of spontaneous seizures. Thus, Nrf2 is one of the most promising target for treatment of the neurodegenerative disorders and epilepsy.

Andrey Y. Abramov is a Professor at the Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology. He studies the role of mitochondria, calcium signalling and redox biology in physiology of the Central nervous system and in the mechanism of the pathology of neurodegenerative disorders. In the last decade in collaboration with Professor Dinkova-Kostova we identified novel and underestimated role of Nrf2 in mitochondrial bioenergetics
- 13:00 - 14:00 Breakout rooms and lunch.
- 14:00 - 15:45 SESION 6. Chair: Gina Manda
NF1086: an NRF2 inducer with potential interest in Alzheimer´s
Manuela G. Lopez, E. del Sastre, C. Fernández Mendivil and I. Rodriguez-Franco
Department of Pharmcology. Medical School and Institute Teofilo Hernando. Autonomous University of Madrid, Spain
Email: m.garcia@uam.es
This study has been developed within a multidisciplinary project designed to search for multitarget compounds with NRF2 inducing capacity, by inhibiting the Keap1-NRF2 interaction, for the treatment of Alzheimer’s disease. We have focused on melatonin derivatives, which additionally induce the NRF2 transcription factor, master regulator of oxidative stress. NF1086 is a melatonin derivative which duplicates NRF2 expression at a concentration below 1 µM, has antioxidant properties and is predicted to cross the BBB through the PAMPA test. Further on, in vivo target validation was assessed by measuring NRF2 and some of its target genes in brain samples after peripheral administration. To test the potential effects of NF1086 in tauopathy, primary cortical neuronal cultures were treated with adeno-associated particles which express the human tau protein with P301L mutation (AAV-hTauP301L). In this in vitro model, phosphorylated Tau was significantly reduced in cultures treated with NF1086. We next evaluated if the compound could provide neuroprotective effects in vivo. Tauopathy in adult (3-4 months) C57bl/6 mice was generated by injecting stereotaxically AAV-hTauP301L in both hippocampi; mice were treated with 20 or 50 mg/Kg/day orally for 35 days, starting after induction of tauopathy. NF1086 treatment prevented cognitive decline promoted by tauopathy. Moreover, it reduced tau hyperphosphorylated protein in different brain regions and reduced neuroinflammation. Interestingly, similar results were achieved in aged mice 20 months old.
In conclusion, NF1086 is a melatonin derivative that induces NRF2 with neuroprotective properties with potential therapeutic interest in tauopathy and related NDDs like Alzheimer´s disesase; however, future studies need to be conducted to elucidate its mechanism of action.

Manuela G. Lopez is MD PhD and full professor of Pharmacology at the Department of Pharmacology in the School of Medicine, Universidad Autonoma de Madrid (UAM) Spain. Currently, she heads the Institute Teofilo Hernando for drug discovery (http://www.ifth.es/) that belongs to UAM. Her group, “NeuroprotectionLab”, has particular interest in the identification of new potential therapeutic targets to develop innovative and disease modifying therapies for neurodegenerative diseases, with special focus in modulating neuroinflammation (microglia-astrocyte interaction), oxidative stress and autophagy. Within the field of NRF2, she has contributed to the understanding of NRF2 in pain, depression, stroke and neurodegenerative diseases, together with the development of different NRF2 multitarget drugs in collaboration with medicinal chemists. Currently, she is coordinating a drug development project to identify Keap1-NRF2 inhibitors with potential use in Alzheimer’s disease.
Dipeptidyl peptidase 3, competitive interactor of KEAP1 protein
Mihaela Matovina
Division of Organic Chemistry and Biochemistry, Ruđer Bošković Institute, Zagreb, Croatia
Email: mmatovina@irb.hr
Dipeptidyl peptidase 3 (DPP3) is a metallopeptidase that cleaves dipeptides from the amino-termini of 3 to 10 amino acids long peptides. It has a likely role in the final stages of protein turnover in the cell and, by cleaving bioactive peptides, probably has a role in the regulation of blood pressure and pain. Overexpression of DPP3 has been found in cancers of various etiologies, and recently it has also been considered as a biomarker in cardiogenic shock, however, the putative mechanisms of its pathophysiology are still largely unclear. DPP3 has also been identified as one of the interactors of KEAP1, acting as a competitive inhibitor of the KEAP1-NRF2 complex, and activator of the KEAP1-NRF2 pathway. We are investigating the interaction of DPP3 and the Kelch domain of the KEAP1 protein using molecular modeling, biochemical and biophysical methods to determine the mechanism, affinity, and thermodynamic parameters of binding. DPP3 binds the Kelch domain of the KEAP1 protein via its ETGE motif in a similar manner to NRF2. Molecular dynamics simulations of the DPP3-Kelch complex have shown that the ETGE motif of DPP3 is located on the flexible loop anchored to the protein body by H-bonds between Glu480 and Arg623 or Arg624 and that the ETGE loop must be released from the protein body for binding to occur. Additional evidence that release of the ETGE loop is essential for binding was provided by microscale thermophoresis (MST) analysis of the interaction of the mutant variant DPP3-R623W (found in the cBioPortal for Cancer Genomics). The results of the combined computational and experimental studies confirm that one of the mechanisms of DPP3 involvement in cancer progression may be through upregulation of the KEAP1-NRF2 pathway.

Mihaela Matovina is the Research Associate in the Laboratory for Protein Biochemistry and Molecular Modeling (LBPMM) at the Division of Organic Chemistry and Biochemistry of Ruđer Bošković Institute in Zagreb, Croatia, and Assistant Professor at Josip Juraj Strossmayer University of Osijek, Croatia. LBPMM is investigating the physiological role of dipeptidyl peptidase 3 protein, including its role in the regulation of the KEAP1-NRF2 pathway. Our current investigations are focused on experimental and computational analysis of KEAP1-DPP3 interaction, as well as the effects of DPP3 mutations found in cancer on the interaction with KEAP1 and the expression of NRF2-controlled genes.
Microenvironmental Nrf2 supports growth of the orthotopic pancreatic tumors and modulates the efficacy of chemotherapy
Monika A. Jakubowska
Malopolska Centre of Biotechnology, Jagiellonian University in Krakow, Poland
Email: monika.jakubowska@uj.edu.pl
Malignant transformation of the enzyme-producing pancreatic secretory epithelia and the development of pancreatic cancer (PC) are largely driven by the activating mutations in the proto-oncogene KRAS, which in turn induces strong antioxidant protein response mediated by NRF2. Importantly, solid pancreatic tumors are characterized by a very prominent fibroinflammatory reaction and the development of the specific tumour microenvironment of non-epithelial origin. In the most extreme cases desmoplasia in PC can account up to 80% of the tumor mass. This progressive fibrosis in tumor microenvironment impairs the efficacy of all therapies against PC. While the antioxidant redox programme has been suggested to play a role in this process, it is still not entirely clear whether it is protective or may worsen the progression of the disease. By using an orthotopic model of a mouse pancreatic tumor grown in the pancreata of the wild-type (WT) and Nrf2 transcriptional knock-out (tKO) mice, we showed that the active microenvironmental redox programme in the WT tumor hosts supports growth of pancreatic tumors, increases tumor burden and causes earlier symptoms of multiorgan failure than in the tKO counterparts. What is more, loss of transcriptionally active Nrf2, improves the efficacy of anti-cancer treatment. Taken together, our study provides a rationale for combining (chemo)therapy of PC with modulation of Nrf2 pathway in order to achieve better therapeutic outcomes against currently incurable pancreatic cancer.
Monika Jakubowska is an Assistant Professor in Cell Physiology/Cell Signalling in the group Molecular Mechanisms of Disease, and a Research Director of the ABSL3+ Animal Unit, both at the Malopolska Centre of Biotechnology, in Krakow, Poland. Together with her coworkers, Monika investigates the role of redox imbalance in pancreatic (patho)physiology, e.g. pancreatic cancer. Other Monika’s interests centre on transition metal ions, mainly copper and iron, in (patho)physiology of the gastrointestinal tract, as well as animal models of cancer, sterile inflammation and infectious human and animal diseases.
Nrf2 signaling in health and disease
Niki Chondrogianni
Institute of Chemical Biology, National Hellenic Research Foundation, Athens, Greece
Email: nikichon@eie.gr
Ageing is a complex process affected by both genetic and environmental factors, characterized by a gradual failure of functionality, reduced stress response and resistance, leading to enhanced probability for age-related diseases and mortality. Proteasomes are constituents of the cellular proteolytic network that maintain protein homeostasis through regulated proteolysis of normal and abnormal (in any way) proteins. Proteasome activation in human primary cells and the model organism Caenorhabditis elegans has resulted in cellular and organismal lifespan extension and in deceleration of protein aggregation in Alzheimer’s (AD) and Huntington’s (HD) nematode models. SKN-1, the homolog of Nrf2 in C. elegans has been at least partially implicated in the regulation of these positive effects. A dietary triterpenoid, namely 18α–glycyrrhetinic acid (18α-GA) that activates SKN-1/Nrf2, has been shown to promote similar beneficial effects in human primary cells and in C. elegans. Lately, we have also shown that the same Nrf2 activator protects human primary cells from MMC-induced genotoxicity through the ERK/Nrf2 pathway. Our results reveal an additional beneficial effect of the Nrf2 activator 18α-GA, suggesting that this important phytochemical compound is a potential candidate in preventive and/or therapeutic schemes against conditions (such as aging) or diseases that are characterized by both oxidative stress and DNA damage. Our work identifies new bioactive compounds with anti-ageing and/or anti-aggregation properties or reveals additional beneficial properties on already known bioactive compounds.

Niki Chondrogianni is a Director of Research at the Institute of Chemical Biology, National Hellenic Research Foundation. She focuses on the genetic and environmental factors that govern ageing, longevity and age-related diseases with emphasis on proteasome regulation. She is seeking for compounds that may act as proteasome activators and thus may serve as anti-ageing agents while dissecting at the same time the involved molecular pathways. She is equally interested in identifying compounds than can decelerate protein aggregation and thus the progression of proteinopathies with emphasis on Alzheimer’s disease using C. elegans as a model in combination with human cells of neuronal origin and murine primary neurons. Various molecular pathways have been revealed to be involved in such beneficial results including the Nrf2/SKN-1 pathway. She is a national and international patent holder that resulted in the development of novel anti-ageing products that act through the activation of the proteasome system (two relative product series in the national/international market).
The bile acid ethylidene derivatives as valuable compounds for the potential treatment of NRF2-related diseases. Synthesis and biological activity.
Srđan Bjedov
Department of Chemistry, Biochemistry and Environmental Protection, Faculty of Sciences, University of Novi Sad, Serbia
Email: srdjan.bjedov@dh.uns.ac.rs
The nuclear factor erythroid 2-related factor 2 (NRF2) participates in multiple important homeostatic functions including regulation of metabolism, inflammation, immune responses, and proteostasis. The pleiotropic role and wide expression in the tissue make NRF2 an invaluable pharmacological target for the treatment of inflammatory, neurodegenerative, metabolic, and cancer diseases. Synthetic steroids derived from oleanolic acid Bardoxolone and Omeveloxone are in late clinical phases for treatment of NRF2 related diseases but with signs of cardiovascular safety issues, and it is suggested that bile acid—ursodeoxycholic acid, an FDA-approved drug for the treatment of primary biliary cirrhosis, owes part of the therapeutic activity to interaction with NRF2. We speculate that bile acid ethylidene derivatives, developed by our group, could be valuable compounds for the development of potential drugs for the treatment of NRF2-related diseases. Here, we want to present you synthesis and biological study of bile acid, amide and oxazoline ethylidene derivatives.
Srđan Bjedov is an assistant professor at the Department of Chemistry, Biochemistry and Environmental Protection, Faculty of Sciences, University of Novi Sad, Serbia. His research is focused on the synthetic and medicinal chemistry of mainly steroid compounds for the treatment of inflammatory, microbial, and cancer diseases.
The Protective Role of NRF2 Signaling Against NLRP3 Inflammasome Activation in Brain
Kemal Ugur Tufekci
Centre for Brain and Neuroscience Research, Izmir Democracy University, Izmir, Turkey
Email: kemalugur.tufekci@idu.edu.tr
The NLRP3 inflammasome is a multiprotein complex that activates caspase-1 and triggers the release of the proinflammatory cytokines IL-1β and IL-18 in response to diverse signals and consequent cell death, named pyroptosis. Although inflammasome activation plays critical roles against various pathogens in host defense, overactivation of inflammasome contributes to the pathogenesis of inflammatory diseases, including psychiatric conditions, acute brain injuries, and chronic neurodegenerative disorders. Microglial cells are the primary mediator of NLRP3 inflammasome activation in the brain. Therefore, our studies are mainly related to microglial immune responses in cell culture and animal models. To test the protective role of NRF2, we have utilized protective molecules with NRF2 activating capacities, such as Sulforaphane, Melatonin, and Dimethyl Fumarate. Our studies have revealed that Sulforaphane, Melatonin, and Dimethyl Fumarate protected microglial cells from NLRP3 inflammasome activation and pyroptotic cell death. On the other hand, using a small molecule inhibitor or siRNA of NRF2 reversed the protective roles of Sulforaphane, Melatonin, and Dimethyl Fumarate. Thus, we conclude that NRF2 signaling mediates the protective functions of Sulforaphane, Melatonin, and Dimethyl Fumarate against NLRP3 inflammasome activation in the brain.

Kemal Ugur Tufekci is an assistant professor of Molecular Neurobiology at the Izmir Democracy University of Turkey. He studies the neuroinflammatory mechanisms involved in the pathogenesis of neuropsychiatric diseases. In recent years, he has focused on the protective role of the NRF2 transcription factor in NLRP3 inflammasome activation associated with neuropsychiatric disorders by utilizing in vivo and in vitro models.
Targeting Nrf2 to control CD4+ T cells contribution to the gut- brain axis
Michel Edwar Mickael
Department of experimental genetics, institute of genetics and animal biotechnology, Polish academy of science, Warsaw, Poland.
Email: m.mickael@igbzpan.pl
The role of NRF2 in mediating the role CD4+ T cells in the gut-brain axis is not yet clear. The gut- brain axis plays a fundamental role in various diseases associated with the gut such as IBD, colitis and cancer as well as neuroinflammatory diseases such as Alzheimer’s and Parkinsons. Current research focuses on the role of the vagus nerve in controlling the communication between the gut and the brian. However, drugs targeting this nerve activity have been shown to be not sufficient for controlling the gut brain communication. We have discovered that CD4+ T cells migrate from the gut to the brain during inflammatory conditions. We saw that once inside the brain, CD4+ T cells can cause demyelination and astrogliosis and are associated with cognitive and psychological manifestations such as depression. However the role of NRF2 in mediating this relationship is still not clear. Our research is focused on understanding the NRF2 role in mediating the role of CD4+ T cells. In various neuroinflammatory diseases, lower levels of NRF2 have been associated with higher expression of inflammatory cytokines such as IL17A and IL17F. IL17A and IL17F are known to be produced by pathogenic CD4+ T cells. Thus our main hypothesis is that NRF2 is capable of inhibiting pathogenic CD4+ T cell migration while supporting anti-inflammatory T cells extravasation. Supporting our hypothesis, we and others found that activating NRF2 resulted in decreasing inflammation along with decreasing depression symptoms in mice
Michel is an assistant professor of neuroimmunology at the Polish academy of science at the department of experimental genomics. He studies the molecular mechanisms involved in the interaction between CD4+ T cells and the neural and glial initiation and progression of neurodegenerative diseases in connection with the gut. He is interested in i) understanding the role NRF2 in CD4+ differentiation, reprogramming and migration. ii) Development of new NRF2-modulating drugs that can decrease inflammation and hence improve neurodegenerative disease prognosis.
- 15:45-16:00 Coffee break, Breakout rooms
- 16:00-17:30 SESION 7. Chair: Manuela G. López
Phytohormones Strigolactones: Their Promising Therapeutic Roles in Inflammation Related Noncommunicable Diseases through Modulating NRF2-KEAP1, BDNF-TrkB and NLRP3 Signaling Cascades
Tugba Boyunegmez Tumer
Department of Molecular Biology and Genetics, Faculty of Arts and Science, Canakkale Onsekiz Mart University, Canakkale, 17020 Turkey
Email: tumertb@gmail.com, tumertb@comu.edu.tr
Strigolactones (SLs), subclasses of structurally diverse and biologically active carotenoid-derived lactones (also known as apocarotenoids), have been identified as new phytohormones. The studies for the bioproperties of SLs as phytohormones date back to the 2010s, thereby; the number of investigations evaluating the potential biomedical promises of SLs in mammalian is limited. It is well established that phytohormones not only govern important physiological traits in plants but also have impacts on human physiological and pathological processes such as cell division, glucose metabolism and inflammation. In fact, uncontrolled and persistent systemic inflammation may develop into a chronic state that finally becomes unifying seed for the pathogenesis of various noncommunicable diseases such as neurodegenerative disorders, insulin resistance and even cancer. Our team has identified interactions of SLs with several signaling molecules and enzymes involved in inflammation, glucose metabolism, and cytoprotection within mammalian systems by using both in vitro and in silico analysis. In these studies, we showed that a representative SL analog, GR24 induced the expression of phase II detoxifying enzymes by activating Nrf2 in hepatic and macrophage cells under normal and inflammatory conditions. Nrf2 silencing and in silico molecular docking (against 16-mer peptide binding site on Keap1) studies suggested that GR24 may exert its anti-inflammatory activity by interfering with Keap1 and Nrf2 binding. Very recently, we have also showed that SLs have the potential for transcriptional regulation of Nrf2 mediated antioxidant pathway in brain microglia and endothelial cells of cerebral micro vessels. In this presentation, in addition to above-mentioned findings, we would like to discuss our current data demonstrating SL analogs with different bioactiphores can be multi-potent glia-and neuroprotective agents and in addition to Nrf2-Keap1 pathway they can be considerably effective against several neuroinflammation-related signaling cascades such as BDNF-TrkB and NLRP3. Therefore, SLs may provide novel multipotent therapeutically active skeleton on which in silico optimized SL-like pharmacophores can be developed and synthesized against inflammation related noncommunicable diseases for further preclinical and clinical investigations.

Tugba Boyunegmez Tumer is Full Professor of Biochemistry at the Department of Molecular Biology and Genetics, Çanakkale Onsekiz Mart University, Turkey. Her research team has mainly focused on understanding the molecular effects of natural product-derived and synthetic small molecules on chronic inflammatory diseases and associated degenerative conditions, particularly, cancer and neuroinflammation. Her lab combines in vitro/in vivo assays, multi-omic tools and in silico computation, modelling and ADMET approaches for rational lead discoveries. Working on the interface of molecular pharmacology, molecular biology, and biochemistry, NRF2-KEAP1 signaling pathway stands at the center of her studies. She has been working on Strigolactones (SLs) and their potential health benefits in chronic inflammatory diseases since 2016 in the scope of EUROCAROTEN Cost action. Besides, she has moved her interest in design and synthesis of H2S releasing compounds including novel isothiocyanate derivatives as NRF2 activators. Prof. Tumer is the leader of several national projects with international collaborators, and she is working group member of Cost Actions CA15136 and CA20121.
Flavonoids – mere antioxidants or specific ligands for biological targets??
Vladimír Křen
Laboratory of Biotransformation, Institute of Microbiology of the Czech Academy of Sciences, 14220 Prague, Czech Republic
Email: kren@biomed.cas.cz
Flavonoids are considered to have strong antioxidant and antiradical activity. They are in fact poor antioxidants in vivo. It is well documented that herbal “antioxidants” cannot achieve in vivo the postulated effects. Nevertheless, numerous beneficial biological effects of flavonoids cannot be denied. This paradox can be well explained by the concept of “parahormesis“, which assumes that “antioxidants” act prooxidatively to activate nuclear factor Nrf2, which then triggers the synthesis of “true antioxidants” in the cell, e.g., enzymatic cytoprotective systems. Proper interaction with the receptor requires the corresponding 3D structure of the ligand is required. Flavonolignans from milk thistle (Silybum marianum) are characterized by the presence of two to four chiral centers (Figure 1). They occur in nature as different stereomers, and numerous recent studies have demonstrated that stereochemistry plays a fundamental role in describing their biological effects. We have developed a highly efficient enzymatic method for preparatory isolation of other silymarin flavonoids, such as silychristin A, silydianin, and dehydroflavonolignans. Together with advanced chemical and enzymatic methods, we have in hands a large library of optically pure flavonolignans, semisynthetic derivatives, and their metabolites that can be tested in various biological models in this Action. Typically, silychristin A was shown to activate the estrogen receptor-α-dependent Nrf2-heme oxygenase-1/superoxide dismutase 2 (Nrf2-HO-1/SOD2) pathway to decrease apoptosis and upregulate glucagon-like peptide-1 (GLP-1) production in intestinal GLUTag L cells, leading to the reversal of reactive oxygen species-induced apoptosis and impairment of GLP-1 production. Silychristin A thus contributes to the achievement of stable physiological glucose homeostasis. We will show that the specific activities of the respective diastereomers of flavonolignans differ significantly in the 3D anisotropic systems (typically biological systems). In vivo, silymarin flavonolignans do not act as redox antioxidants, but they play a role as specific ligands of biological targets according to the “lock-and-key” concept.

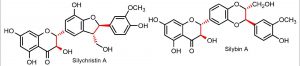

Vladimír Křen is Senior researcher in the Laboratory of Biotransformation, Institute of Microbiology of the Czech Republic in Prague and a full Professor of Medical Chemistry and Biochemistry at Palacký University, Olomouc. Long-time expert in the chemistry and chemoenzymatic transformation of flavonolignans of silymarin, in the biotransformation of natural products by enzymes and microorganisms, but also in glycosidases and glycosyltransferases, pharmacology of glycosides, glycobiology. 370 publications, h = 46; ORCID: 0000-0002-1091-4020
Increased ROS can improve cell survival: stress response PACOS
Irina Milisav
Department of Pathophysiology, Faculty of Medicine, University of Ljubljana, Slovenia
Faculty of Health Sciences, University of Ljubljana, Slovenia
Email: irina.milisav@mf.uni-lj.si
Moderate concentrations of reactive oxygen species (ROS) are required for normal cell function, while excessive or too small amounts of ROS lead to oxidative imbalance, named oxidative stress or antioxidative/reductive stress, respectively. Cells are exposed to many stressors in vivo and in vitro, to which they respond with signal cascades and metabolic adaptations. H2O2 is the main redox signalling and redox regulation molecule and its interactions can modulate the activity of mammalian transcription factors, including NRF2.
Increased levels of H2O2 are a part of a normal response to moderate stress in primary liver cells (hepatocytes) and trigger preapoptotic cell stress response (PACOS), which results in decreased apoptotic triggering through the mitochondrial pathway. Both adaptations, increased amount of ROS production and lower apoptosis triggering – PACOS, revert to the levels similar in the liver after some days. The function of hepatocytes is preserved at all times; in both, stress adapted and normal culture cells. Antioxidants, like N-acetylcysteine (NAC), annuls the PACOS stress response and its protective role against apoptosis.

Irina Milisav is a full professor of Biochemistry and Molecular Biology at University of Ljubljana, Slovenia, employed at Faculty of Medicine, Institute of Pathophysiology as a Research Associate and at Faculty of Health Sciences on a teaching post. She studies adaptive stress responses that boost cellular defences in liver cells (hepatocytes). Her group discovered an adaptive stress response of hepatocytes that prevents unnecessary apoptosis triggering when the stress-adapted hepatocytes are exposed to subsequent moderate stress (PACOS). ROS signalling mobilizes antioxidative defence and PACOS. Her group also investigates the effects of selected second generation antipsychotics on the liver as a part of a Marie Sklodowska Currie ITN project and the role of stress responses in drug-induced liver injury (DILI).
The nanotechnology to improve the treatment of Alzheimer’s and other neurological diseases
Joana A. Loureiro, Maria do Carmo Pereira
LEPABE – Laboratory for Process Engineering, Environment, Biotechnology and Energy, Faculty of Engineering, University of Porto, Porto, Portugal
Email: mcsp@fe.up.pt; jasl@fe.up.pt
Alzheimer´s Disease (AD) is the most common form of dementia with high impact worldwide, accounting for more than 46 million cases. Until now there is no cure for AD. Moreover, growing life expectancy will lead to an increase in numbers: to 75 million by 2030, 81.1 million by 2040 and 132 million in 2050 all over the world.
Most of the AD treatments shows limitations mainly related with its low bioavailability, requiring high administration doses of oral administration, to reach the brain at sufficient concentrations. The use of drug delivery systems (DDSs) based on nanoparticles (NPs) could be the ideal solution to overcome these issues. NP based DDSs can protect the molecules from degradation, transport them as “Trojan horses” and improve biodistribution, without modifying the therapeutic activity. NP-DDSs have several beneficial properties, such as biodegradability, biocompatibility, nontoxicity, non-immunogenicity, high stability in body fluids, and the ability to interact with specific receptors. In addition, the NPs allow a controlled and sustained drug release, facilitating the maintenance of therapeutically active doses for prolonged periods. Besides, drug encapsulation into nanocarriers reduces the required doses to produce the expected therapeutic effects due to an increased concentration at the site of action. In the last years, our team developed different types of DDS, such as liposomes, polymeric poly(lactic-co-glycolic) acid (PLGA) NPs and lipid nanoparticles, to encapsulate therapeutic molecules.
Transcytosis mediated NPs transportation by specific receptors is the most relevant mechanism to transport drugs across the blood-brain barrier (BBB). The conjugation of specific ligands on the NPs’ surface can improve their transfer. The appropriate selection of the ligand is crucial to increase the transport efficiency of NPs since the receptor needs to be overexpressed at the target site. That way, our group has a know-how to modulate the NPs surface to improve their uptake in the desired tissues.

Maria do Carmo Pereira completed the PhD in Chemical Engineering in 1998 at the University of Porto, Faculty oe Engineering (FEUP), and a graduation in Chemical Engineering in 1993 at the same university. She is Associate Professor at FEUP and the Group Leader of Supramolecular Assemblies at the Laboratory for Process Engineering, Environment, Biotechnology and Energy (LEPABE). The main research areas that she is working are: i) Nanotechnology and interfacial phenomena; supramolecular interactions including novel nano-engineered biomaterials for therapeutic applications; development of nanostructured electrochemical immunosensors for detection of neurodegenerative diseases.

Joana A. Loureiro is a researcher at the Laboratory for Process Engineering, Environment, Biotechnology and Energy (LEPABE) since 2010 and Invited Assistant Professor at Faculty of Engineering, University of Porto (FEUP) since 2018. Joana did her bachelors and masters in chemical engineering followed by a bachelors and masters in pharmaceutical sciences. She received her PhD degree in chemical and biological engineering from FEUP in 2013. Since 2010, Joana has been working in the field of drug delivery nanosystems for brain diseases treatment. She has expertise in protein misfolding and pathogenic biomarkers associated with Alzheimer’s disease. Her main research areas of interest comprise: i) nanotechnology and interfacial phenomena; ii) effects of fluorinated systems and peptides on the aggregation of amyloid-beta peptide; iii) conformational studies of proteins and peptides self-organized systems and polymer surfaces; and iv) design and production of inorganic and polymeric nano-systems for pharmaceutical application..
Cholesterol Mediated Nrf2 Status in NASH Development: Role of α-Tocopherol
Nesrin Kartal Ozer
Department of Biochemistry, Faculty of Medicine, Marmara University, Maltepe, Istanbul, 34854, Turkey
Email: nkozer@marmara.edu.tr
Nonalcoholic fatty liver disease (NAFLD) is a metabolic disorder characterized by excessive fat accumulation in the liver due to disturbed hepatic cholesterol homeostasis. Oxidative stress, inflammation and apoptosis driven by enhanced fat accumulation are critical mechanisms involved in the progression from NAFLD to nonalcoholic steatohepatitis (NASH). Even though, it is of utmost interest to understand the precise regulatory mechanisms of lipid accumulation in the human liver, underlying mechanisms leading to the transition from NAFLD to NASH are not fully understood. Against oxidative stress and inflammation, Nrf2 signaling involves in the regulation of a number of genes, including enzymes and cytoprotective proteins.
In our hypercholesterolemic rabbit model, high cholesterol diet induced 4-hydroxy-2,3-nonenal (HNE), 7-ketocholesterol (7KC) and Nrf2 levels along with other inflammation and apoptosis parameters, which involve in NASH progression. This is also consistent with our previous reports demonstrating that high cholesterol diet mediated HNE and Nrf2 induction in atherosclerosis development. Alpha-tocopherol, the most active form of vitamin E, is an important modulator of signaling mechanisms, but its involvement to cholesterol-mediated Nrf2 induction remains poorly defined. We showed that α-tocopherol supplementation reduced only protein levels of Nrf2 without affecting mRNA in hypercholesterolemic animals. Although α-tocopherol supplementation caused significant reduction of HNE, 7KC and Nrf2, lipid accumulation was not changed. These findings lead us to conclude that the steatosis-related beneficial effect of α-tocopherol in liver tissue is rather limited.

Nesrin Kartal Ozer is Professor at Marmara University, Faculty of Medicine, Department of Biochemistry, Istanbul,Turkey. She graduated with a BSc in Pharmacy from Hacettepe University, Ankara, Turkey and she has received her PhD in Biochemistry in Hacettepe University, Faculty of Medicine. She moved then to Marmara University, Istanbul. She was a Visiting Scientist at St.George’s Hospital, Medical School, Department of Biochemistry, London, UK (1985-1986); Institute of Biochemistry and Molecular Biology, University of Bern, Switzerland (1993-2004) and University of Hohenheim, Stuttgart, Germany (2008).
In her research she published ~ 100 articles and she was invited speaker at ~80 international meetings. She was the organizer ~10 scientific meetings and conferences.
As member of International Organizations, it is worth mentioning her membership in the International Advisory Committee, Oxygen Club of California (1999- today); FEBS Advanced Course Committee Member (2003-2006); FEBS Fellowships Committee Member (2021-2024), Secretary General Society for Free Radical Research-Europe (SFRR-E) and Member of the Council (2003-2009) and President of SFRR-E (2013-2014).
She was awarded The Oxygen Club of California Award and “Life Time Honorary Member” in 2006 “In recognition of outstanding research contributions on the role of vitamin E in atherosclerosis and to fostering the field of free radical biology” and The Society for Free Radical Research-Europe Lifetime Achievement Award in 2019 “In recognition of Lifetime Extraordinary Scientific Achievements in the Field of Free Radical Research”.
Her research focus on endoplasmic reticulum stress, redox signaling and cell death in metabolism related diseases such as cardiovascular diseases and non-alcoholic fatty liver disease. In parallel she has carried out research on the molecular function of tocopherols in these diseases.
Nrf2 signaling: a double-edged sword in metals toxicity
Aleksandra Buha Djordjevic
Department of Toxicology, University of Belgrade – Faculty of Pharmacy, Belgrade, Serbia
Email: aleksandra@pharmacy.bg.ac.rs
Human exposure to various pollutants, ubiquitously present in the environment, mainly due to anthropogenic activities has been implicated in the development of many human diseases. However, exact toxic mechanisms of these pollutants remain scarcely clarified. Nuclear factor erythroid 2-related factor 2 (Nrf2), an emerging regulator of cellular resistance to oxidants, serves as one of the key defensive factors against a range of pathological processes such as oxidative damage, carcinogenesis, all implicated in toxicity of various environmental toxicants. However, conflicting data have been obtained so far regarding the interplay between environmental toxicants and Nrf2 signaling. While some results indicate that the exposure can lead to the increase in Nrf2 activation by Keap1- mechanisms, others reveal that the Nrf2 defence pathway is inhibited under the influence of such exposure. The outcome is, nevertheless, largely depended on the duration of exposure, with Nrf2 signalling pathways playing a significant defence role in the conditions of shorter exposure to toxicants, while chronic exposure seems to provide a favorable environment for cell survival and tumorigenesis due to hyperactivation of Nrf2 signalling pathways. Thus, our future animal studies are aiming to expand the body of knowledge regarding the enigmatic interrelations between the various environmental pollutants and Nrf2 signaling.

Aleksandra Buha Djordjevic is an associate professor of Toxicology at the Department of Toxicology, University of Belgrade – Faculty of Pharmacy. Her main research interest is toxicology of mixtures and endocrine disrupting chemicals. In the field of NRF2 signalling, her special interest is towards the role of this pathway in the toxicity of environmental toxicants. She is the PI of the national project Decoding the role of exposome in human endocrine health funded by The Science Fund of the Republic of Serbia. She is author/co-author of more than 60 peer-reviewed publications, her h index is 22 and her work has been cited more than 1400 times according to SCOPUS. Dr. Buha Djordjevic has been included in the Stanford University list of top 2% scientists in the world for 2020.
- 17:30-18:00 Breakout rooms
- 18:00-18:30 Round table and Concluding remarks