Non-communicable diseases (NCDs) account for 77% of all deaths in Europe and remain the most prevalent and without effective therapy. Networking among multidisciplinary teams that explore disease from a perspective of causative pathomechanisms rather than clinical symptoms is the most appropriate approach to overcome this problem. Such pathomechanisms imply the loss of homeostatic functions leading to the pathologic formation of reactive oxygen species, chronic inflammation, metabolic unbalance and proteinopathy.
The transcription factor NRF2 is a master regulator of multiple cytoprotective responses and a key molecular link among many NCDs. It provides a unique strategy for drug development and repurposing that is now starting to be translated to the pharmacological and clinical arena.
This Action will build a network of excellence for integrating and spreading the existing knowledge and providing innovative services, drugs and tools related to NRF2-pharmacology, with the final goal of boosting the translation to the European industry sector.
NRF2 as a master regulator of cytoprotective responses. (a) Nrf2 (NF-E2-related factor 2), a member of the Cap’n’collar (CNC) transcription factor family, consists of 605 amino acids and is divided into seven highly conserved functional domains, known as Neh1-Neh7. (Source: Saha S et al., 2020 Molecules). (b) NRF2 heterodimerizes with the members of MAF family through their bZip domain. The heterodimer binds to an enhancer sequence termed ARE that is present in the regulatory regions of over 250 genes (ARE genes). (c) These genes participate in the control of redox metabolism, inflammation, and proteostasis balance, as indicated. The existence of susceptibility SNPs in NFE2L2, elevated levels of its target genes in brain necropsies, and positive data from preclinical studies suggests that the imbalance in proteostasis,redox, and inflammatory control may be counterbalanced by NRF2 activation.
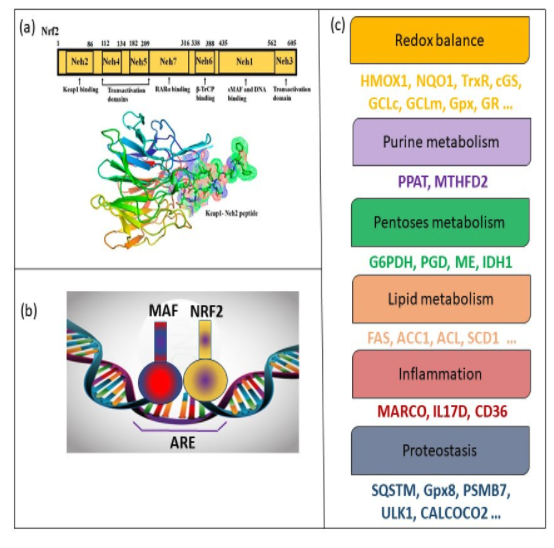
The fact that many NCDs share common pathomechanisms and exhibit a high degree of molecular connectivity is supporting a new concept of disease where a common molecular target may provide, at least partially, therapeutic benefit for several dysregulated cellular responses. One such molecular link is the transcription factor NRF2 (nuclear factor (erythroid-derived 2)-like 2). Evidence gathered for the past 10 years strongly points towards a NRF2-related strategy for drug development and repurposing in these NCDs. This statement has been widely supported for the past years with the Nrf2-knock-out mouse, the “systems medicine” analysis of the role of NRF2 in the connectivity networks among NCDs, and the genetic association between several NCDs and functional polymorphisms in the NRF2 coding gene. Extensive knowledge in the field is now at a mature stage to transform this basic knowledge into social, scientific and clinical awareness and to implement new strategies for drug development and repurposing in those NCDs underlined by low-grade chronic inflammation, oxidative stress, metabolic impairment and proteinopathy, which are the main targets of NRF2.
NRF2 regulates the expression of roughly 250 genes encoding a network of enzymes involved in NADPH-, glutathione- and thioredoxin-mediated reactions, inhibition of inflammation, induction of autophagy genes, etc. Through this transcriptional network, NRF2 coordinates multifaceted responses to diverse forms of stress for maintaining a stable internal environment. The main mechanism of NRF2 regulation is the control of protein stability by KEAP1 (Kelch-like ECH-associated protein 1). Under homeostatic conditions, KEAP1 targets NRF2 for ubiquitin/proteasome degradation. However, electrophiles inhibit KEAP1 and lead to increased NRF2 activity and induction of its target genes. These electrophiles reinforce homeostatic and protective responses through NRF2 activation and provide the basis for drug development. Pharmacological research on NRF2, targeting KEAP1, is very advanced in preclinical models of several NCDs, and is now starting to evolve to the level of clinical practice.
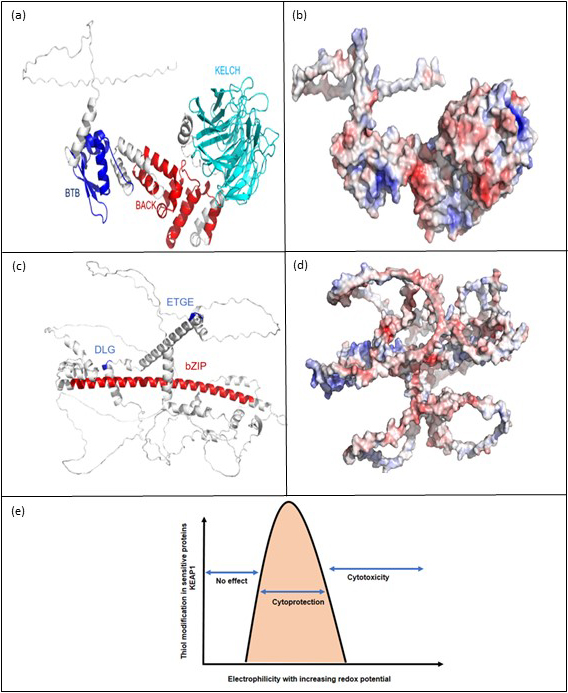
Regulation of NRF2 stability by KEAP1 and its pharmacological targeting. (a) Complete structure of Kelch-like ECH-associated protein 1 (KEAP 1; Source: AlphaFold in silico structure database), Two functional domains (BTB; Position: 77-149 and BACK; Position: 184-286) and repeats region (Kelch1-6 repeats; Position: 327-611) are highlighted. (b) Electrostatic surface potential map of KEAP1 Protein (Display: PyMOL; Blue: Positively charged residues; Red: Negatively charged residues; White: Neutral Residues). (c) Complete structure of nuclear factor erythroid 2-related factor 2 (NRF2) (Source: AlphaFold in silico structure database). The bZIP functional domain is presented at 497-560 residues of NRF2. Two functional motifs: DLG (Residues: 29-31) and ETGE (Residues: 79-82) are also illustrated. (d) Electrostatic surface potential map of NRF2 Protein. Disordered regions are located at 334-449 and 571-605 residues of NRF2. (e) Electrophiles easily modify cysteine residues in KEAP1. The therapeutic window allows KEAP1 modification in the absence of nonspecific thiol modifications within other proteins is represented in the pink area.
NRF2 activators are being used in preclinical and clinical trials of many NCDs where its activity is abnormally low. Two strategies are being used to activate NRF2 in NCDs:
a) Electrophile drugs alter the structure of KEAP1 through the interaction with several cysteine sensors. At least 30 patents are indexed in the World Intellectual Property Organization protecting a variety of molecularly unrelated These and many other compounds have proved to be active in preclinical studies and a few are now in phase 2/3 clinical trials. The case of maximal success is dimethyl fumarate, which is approved for clinical use by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). It is currently used for the treatment of relapsing-remitting multiple sclerosis and psoriasis, and is in phase 2 for rheumatoid arthritis, cutaneous T cell lymphoma and obstructive sleep apnea.
b) Protein-protein interaction (PPI) inhibitors are designed to interfere with the docking of NRF2 onto KEAP1, hence preventing proteasomal NRF2 degradation. Several NRF2/KEAP1 PPI inhibitors are under patent protection.
NRF2 inhibitors hold a great promise in cancer therapy, as its inhibition will result in a significant loss of the capacity of tumor cells to maintain growth and adapt to the hostile tumor microenvironment.
COST (European Cooperation in Science and Technology) is a funding agency for research and innovation networks. Our Actions help connect research initiatives across Europe and enable scientists to grow their ideas by sharing them with their peers. This boosts their research, career and innovation.
Grant Holder: Universidad Autónoma de Madrid
Start of Action: 19 October, 2021
End of Action: 18 October, 2025
CSO approval date: 25 May 2021
Action email: info@benbedphar.org
© 2022 BenBedPhar | Design by Tuinbit Group