NRF2 in COST Action 20121
Our quarterly newsletter attempts to provide our latest news and also aims at becoming a forum for analysis of relevant topics on the field of NRF2 and provide comments to some of the most relevant articles published during the quarter. Previous newsletters can be accessed at:
https://benbedphar.org/our-first-newsletter/
https://benbedphar.org/issue-2-abril-2022/
https://benbedphar.org/issue-3-july-2022/
https://benbedphar.org/issue-4-october-2022/
https://benbedphar.org/issue-5-january-2023/
https://benbedphar.org/issue-6-april-2023/
https://benbedphar.org/issue-7-october-2023/
We are just starting our third grant period. Many exciting activities are scheduled for this year,
including webinars, two training schools, two scientific meetings, etc. In January 19 th , We already had
the first webinar of this year on “NRF2 and Autoimmunity” in collaboration with Sapienza University
wit htwooutstanding speaekrs: Prof. Gerasimos Sykiotis and Dr. Gloria Riitano. It can be accessed at:
https://benbedphar.org/webinar-nrf2-and-autoimmunity/. Be alert to oue webpage for participation in
future events.
Antonio Cuadrado
Chair of COST Action 20121, BenBedPhar
Autonomous University of Madrid
Comments from the Working Groups
NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8
Nuclear factor erythroid 2–related factor 2 (NRF2) is mostly known for its antioxidative function, however it has also been shown to play an oncogenic role in tumour progression by protecting cancer cells against cell death [1,2,3]. Ferroptosis is a type of cell death driven by the accumulation of iron and lipid peroxides. Importantly, cancer cells can evade ferroptosis through NRF2 activation. Taking a step further and expanding this knowledge, Anandhan et al. (2023) could link this protection conferred by NRF2 to the mitigation of both lipid peroxidation and free iron [4]. Which were the key findings leading to this discovery?
Using NFE2L2/NRF2 knockout cells, they showed that these cells had a low expression of NRF2 target gene HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2), therefore leading to a higher stability of F-box and leucine-rich repeat protein 5 (FBXL5) and nuclear receptor coactivator 4 (NCOA4), both targeted for degradation by HERC2. NCOA4 promotes the autophagic turnover of ferritin heavy chain (FTH), while FBXL5 targets the iron regulatory protein 2 (IRP2) for degradation, consequently leading to an increase in the translation of FTH. Therefore, this causes a rise in ferritin and NCOA4, and a concurrent apoferritin (i.e. ferritin that is not bond to iron) recruitment into the autophagosome.
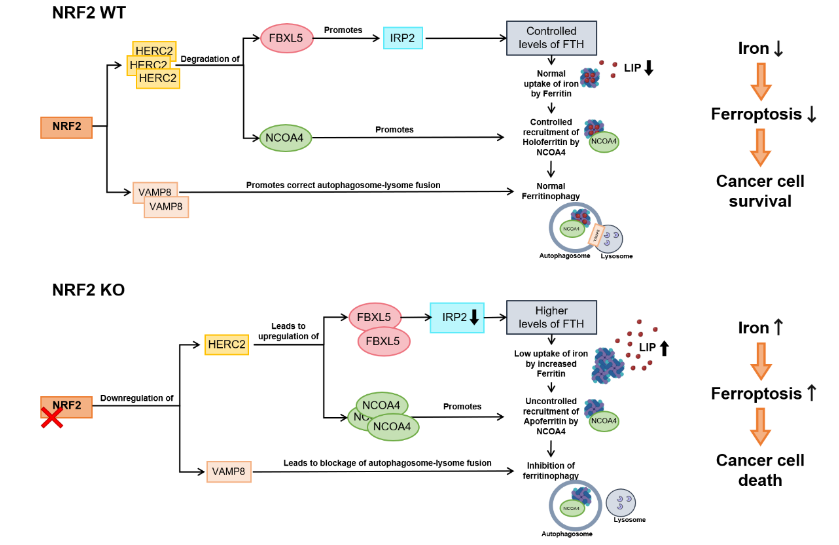
Deletion of NFE2L2/NRF2 results in reduced HERC2 expression, increased stability of FBXL5 and NCOA4, decreased IRP2 protein stability, and enhanced FTH synthesis. In NFE2L2/NRF2 KO cells, decreased transcription of VAMP8 results in blockage of autophagosome-lysosome fusion and inhibition of ferritinophagy. Excessive accumulation of NCOA4 in NFE2L2/NRF2 KO cells causes recruitment of apoferritin into autophagosomes, leading to autophagosomal accumulation of apoferritin/NCOA4, increased LIP, and enhanced sensitivity to ferroptotic cell death. (adapted from Anandhan et al. 2023 paper)
In addition, these cells also presented low expression of vesicle-associated membrane protein 8 (VAMP8), which contributes to a blockage of autophagosome-lysome fusion and inhibition of ferritinophagy, failing to recycle ferritin and leading to its accumulation in the autophagosome. By controlling ferritin synthesis through regulation of HERC2, and the recruitment of ferritin to the autophagosome and consequent degradation via NCOA4 and VAMP8, NRF2 can alter the intracellular labile iron pool (LIP) and dictate the susceptibility to ferroptosis of cancer cells [4]. Additionally, the same study revealed that genetic or pharmacological NRF2 inhibition can enhance the sensitivity to ferroptosis in preclinical models like 3D tumour spheroids, xenografts and ovarian cancer cell lines.
Together, these findings reinforce the need to further explore NRF2 functions and the mechanisms by which it can control iron homeostasis, given the promising results of inducing ferroptosis through the silencing of NRF2 in tumour cells as a therapeutic strategy to treat cancer patients.
References:
- DeNicola, G. M., Karreth, F. A., Humpton, T. J., Gopinathan, A., Wei, C., Frese, K., Mangal, D., Yu, K. H., Yeo, C. J., Calhoun, E. S., Scrimieri, F., Winter, J. M., Hruban, R. H., Iacobuzio-Donahue, C., Kern, S. E., Blair, I. A., & Tuveson, D. A. (2011). Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature, 475(7354), 106–109. https://doi.org/10.1038/nature10189
- Hsu, W. L., Wang, C. M., Yao, C. L., Chen, S. C., Nien, C. Y., Sun, Y. H., Tseng, T. Y., & Luo, Y. H. (2022). Blockage of Nrf2 and autophagy by L-selenocystine induces selective death in Nrf2-addicted colorectal cancer cells through p62-Keap-1-Nrf2 axis. Cell death & disease, 13(12), 1060. https://doi.org/10.1038/s41419-022-05512-2
- Wang, X. J., Sun, Z., Villeneuve, N. F., Zhang, S., Zhao, F., Li, Y., Chen, W., Yi, X., Zheng, W., Wondrak, G. T., Wong, P. K., & Zhang, D. D. (2008). Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis, 29(6), 1235–1243. https://doi.org/10.1093/carcin/bgn095
- Anandhan, A., Dodson, M., Shakya, A., Chen, J., Liu, P., Wei, Y., Tan, H., Wang, Q., Jiang, Z., Yang, K., Garcia, J. G., Chambers, S. K., Chapman, E., Ooi, A., Yang-Hartwich, Y., Stockwell, B. R., & Zhang, D. D. (2023). NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Science advances, 9(5), eade9585. https://doi.org/10.1126/sciadv.ade9585
Mafalda Pita & Rita Carlos
WG1 members
FCUL, Lisbon, Portugal
Inhibiting NRF2 with stapled peptides
The hyperactivation of NRF2 and its role in the hallmarks of cancer has sparked interest in the development of NRF2 inhibitors. This has been a challenging task and most small molecules that have been shown to inhibit NRF2 to date, are in fact inhibitors of global protein translation (1). Inhibiting NRF2 by use of stapled peptides (2) represents an attractive alternative approach.
In 1998, Blackwell and Grubbs (3) reported the synthesis of covalently cross-linked peptide helices by ruthenium-catalyzed ring-closing metathesis. Combining this principle with α,α-disubstitution of the amino acid chiral carbon and on-resin peptide synthesis, Schafmeister et al. (4) were able to achieve enhanced helicity and metabolic stability; such constructs were termed stapled peptides. In contrast to short peptides with natural amino acid composition, which often have high conformational variability, low cell permeability, and high propensity for proteolytic degradation (and thus poor drug-like properties), stapled peptides have compact structures, enhanced cell permeability, and are relatively resistant to proteolysis (2). Furthermore, development of drug resistance is unlikely for stapled peptides, a considerable advantage over traditional small-molecule drugs.
Due to their larger size in comparison with small molecules, stapled peptides are particularly attractive for specifically inhibiting protein-protein interactions. Based on AlphaFold predictions, in a recent study, Modi et al. (5) have developed a stapled peptide (called N1S) that binds to sMAFG and inhibits the protein-protein interactions between NRF2 and sMAFG (Figure 1). At a concentration of 50 μM, N1S inhibits the expression of an NQO1-ARE-luciferase reporter in human embryonic kidney (HEK293) cells as well as the expression of several NRF2-target genes in lung cancer A549 cells with hyperactive NRF2, and sensitizes A549 cells to the chemotherapeutic agent cisplatin.
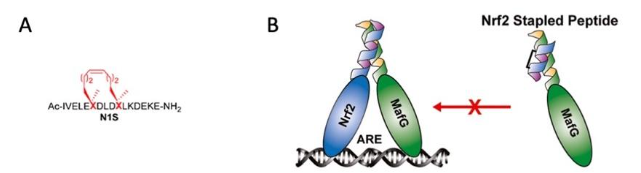
References:
- Harder B, Tian W, La Clair JJ, Tan AC, Ooi A, Chapman E, Zhang DD. Brusatol overcomes chemoresistance through inhibition of protein translation. Mol Carcinog. 2017; 56: 1493-1500.
- Walensky LD, Bird GH. Hydrocarbon-stapled peptides: principles, practice, and progress. J Med Chem. 2014; 57: 6275-6288.
- Blackwell HE, Grubbs RH. Highly Efficient Synthesis of Covalently Cross-Linked Peptide Helices by Ring-Closing Metathesis. Angew Chem Int Ed Engl. 1998; 37: 3281-3284.
- Schafmeister CE, Po J, Verdine GL. An all-hydrocarbon cross-linking system for enhancing the helicity and metabolic stability of peptides. J Am Chem Soc. 2000; 122: 5891-5892.
- Modi R, McKee N, Zhang N, Alwali A, Nelson S, Lohar A, Ostafe R, Zhang DD, Parkinson EI. Stapled peptides as direct inhibitors of Nrf2-sMAF transcription factors. J
Albena
WG2 leader
Duke University, USA
Inhibiting Thiosulfate sulfurtransferase deficiency promotes oxidative distress and aberrant NRF2 function in the brainNRF2 with stapled peptides
Thiosulfate sulfurtransferase (TST, EC 2.8.1.1) was discovered as an enzyme that detoxifies cyanide by conversion to thiocyanate (rhodanide) using thiosulfate as substrate; this rhodanese activity was subsequently identified to be almost exclusively located in mitochondria. More recently, the emphasis regarding its function has shifted to hydrogen sulfide metabolism, antioxidant defense, and mitochondrial function in the context of protective biological processes against oxidative distress. While TST has been described to play an important role in liver and colon, its function in the brain remains obscure. In the present study, we therefore sought to address its potential involvement in maintaining cerebral redox balance in a murine model of global TST deficiency (Tst-/- mice), primarily focusing on characterizing the biochemical phenotype of TST loss in relation to neuronal activity and sensitivity to oxidative stress under basal conditions. Here, we show that TST deficiency is associated with a perturbation of the reactive species interactome in the brain cortex secondary to altered ROS and RSS (specifically, polysulfide) generation as well as mitochondrial OXPHOS remodeling. These changes were accompanied by aberrant NRF2-KEAP1 expression and thiol-dependent antioxidant function. Upon challenging mice with the redox-active herbicide paraquat (25 mg/kg i.p. for 24 h), Tst-/- mice displayed a lower antioxidant capacity compared to wildtype controls (C57BL/6J mice). These results provide a first glimpse into the molecular and metabolic changes of TST deficiency in the brain and suggest that pathophysiological conditions associated with aberrant TST expression and/or activity renders neurons more susceptible to oxidative stress-related malfunction.
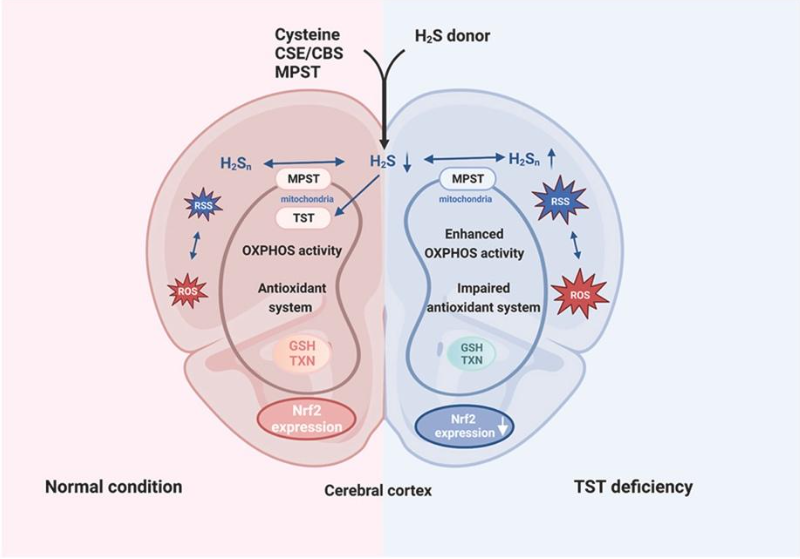
See the full paper (Luo et al., Redox Biology 68 (2023)): https://doi.org/10.1016/j.redox.2023.102965
Harry van Goor
WG3 member
University of Groningen, The Netherlands
The FDA approves an NRF2 activator as the first treatment for Friedreich’s Ataxia, and the EMA reviews the application.
Omaveloxolone, an NRF2 activator, is a synthetic triterpenoid compound being investigated for its potential therapeutic effects in several diseases, particularly those related to inflammation and oxidative stress. It is being developed by Reata Pharmaceuticals, which was recently acquired by Biogen. Omaveloxolone was approved in the FDA in February 2023 for the treatment of Friedreich’s ataxia and is under review by the European Commission, with a final decision expected in the first quarter of 2024. If approved, the therapy will be indicated for adults and adolescents with Friedreich ataxia (FA), aged 16 years and older.

According to Biogen, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion on 14 December 2023, making omaveloxolone the first approved treatment for patients with Friedreich’s ataxia (FA) in the EU. The therapy, which is already approved by the FDA, will continue to be marketed as Skyclarys if approved by the EMA.
FA is a rare neuromuscular disease caused by mutations in the gene encoding the mitochondrial protein frataxin.
Loss of functional frataxin disrupts iron-sulphur cluster biosynthesis, induces mitochondrial dysfunction and increases susceptibility to oxidative stress. FA is a progressive nervous system disorder characterized by mitochondrial dysfunction, impaired NRF2 signaling and reduced energy production. The disease is characterized by uncoordinated muscle movements, poor balance, difficulty walking, changes in speech and swallowing, and a shortened life span. Although the neurological phenotype of FA is well defined, there have been no established pharmacological treatments and omaveloxolone is the first treatment for this pathology.

Omaveloxolone restored mitochondrial function in fibroblasts from patients with the disease in previous experiments and is a promising approach to protect mitochondrial function in these patients.
The treatment label indicates a recommended dosage of 100 mg once daily for people with moderate liver impairment, with a further reduction to 50 mg once daily in the event of adverse reactions. Treatment is not recommended for people with severe liver impairment.
References:
- Mullard A. FDA approves first Friedreich’s ataxia drug. Nat Rev Drug Discov. 2023 Apr;22(4):258. doi: 10.1038/d41573-023-00041-9.
- Wang J, Cao Y, Lu Y, Zhu H, Zhang J, Che J, Zhuang R, Shao J. Recent progress and applications of small molecule inhibitors of Keap1-Nrf2 axis for neurodegenerative diseases. Eur J Med Chem. 2024 Jan 15;264:115998.
Santiago Cuevas
WG4 leader
BioMedical Research Institute of Murcia (IMIB), Murcia (Spain)
NRF2 and Cardio-Oncology: The Difference That the Model Makes
Cancer treatment has evolved greatly over the years, allowing cancer survivorship to reach outstanding rates over the years. Even so, that comes with high costs, as cancer treatments present serious adverse effects. Cardiotoxicity is one of the most prevalent adverse effects among chemotherapeutic agents and even targeted therapies. Indeed, cardiotoxicity has emerged as a leading cause of mortality among cancer survivors: while the probability of dying of cancer decreases with time, the likelihood of dying of cardiovascular disease increases. Indeed, in some ages and cancer types, cardiovascular mortality even surpasses cancer mortality among treated cancer patients.
Doxorubicin (DOX) is a topoisomerase II inhibitor used against several cancers, being a blockbuster drug with a wide use for the last 50 years in cancer therapy. While effective in treating various cancers, DOX poses significant challenges due to its adverse effects, particularly short- and long-term cardiotoxicity. Even so, long-term cardiac remodelling following DOX administration remains poorly understood, as it has been inadequately represented in pre-clinical studies.
We performed two works that aimed assessing the short- and long-term cardiotoxicity of DOX and the role of inflammation and antioxidant defences on that cardiotoxicity in two differently aged mice, adult and elderly male CD-1 mice. Both studies adopted a relevant administration scheme mimicking the clinical use of DOX in humans, involving therapeutic cycles and addressing both short- (1 week) and long-term cardiotoxicity. Regarding the latter aspect, the innovative aspects of both studies included the assessment of long-term cardiotoxicity, conducted months after the last DOX dose, with a 5-month follow-up for adults and a 2-month follow-up for elderly mice.
In adults, DOX at short term (one week), several markers related to inflammation and redox homeostasis (e.g. tumour necrosis factor receptor (TNFR) 2, glutathione peroxidase 1, catalase, inducible nitric oxide synthase (iNOS) cardiac expression, and a trend towards an increase in interleukin (IL)-6, TNFR1, nuclear factor κB (NF-κB) p65 immunopositive cells) increased. Interestingly, both short- and long-term evaluations demonstrated a high density of cardiac infiltrating M1 macrophages induced by DOX. On the other hand, the late effects of DOX (5 months) showed an increase in nuclear NRF2 and superoxide dismutase 2 (SOD2) expression, which was still accompanied by some signs of cardiac inflammation, namely increased levels of myeloperoxidase, IL-33, and tumour necrosis factor-α (TNF-α) expression.
Despite the prevalence of cancer in the elderly population, this demographic is often underrepresented in pre-clinical and clinical studies on cardio-oncology. This limitation is a considerable drawback as it neglects the population most affected by cancer and consequently by chemotherapy-induced cardiac adverse effects. In old mice, DOX induced cardiac histological damage and fibrosis in the short term (1 week post-administration), which persisted even after a 2-month drug-free period. The short-term group given DOX had increased NF-κB p65, iNOS, IL-33 cardiac expression, and a trend toward increased IL-6, indicating inflammation activation. In contrast, animals sacrificed 2 months after DOX displayed significant increases in glutathione peroxidase 1 and Bax expression, coupled with persistent cardiac damage and fibrosis. Notably, Nrf2, NF-κB p65, and myeloperoxidase expression decreased 2 months after the last DOX administration, suggesting a discordant response compared to adult animals. In fact, the adult heart demonstrated the ability to activate redox homeostasis responses even 5 months after DOX exposure, whereas the elderly were not able to activate Nrf2 and other key antioxidant responses that would counteract the inflammatory response seen. This lack of adaptability of the old heart can pose a higher risk for that population to develop cardiovascular diseases, corroborating what is seen on pharmacovigilance studies. These works demonstrate that personalized protective medicine adjusted to the demographic features of the affected population is of key importance on cardio-oncology.
Vera Marisa Costa
WG5 member
UCIBIO – Faculty of Pharmacy University Porto, Portugal
Hot from Pubmed
Immunological dimensions of neuroinflammation and microglial activation: exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression
The increasing life expectancy has led to a higher incidence of age-related neurodegenerative conditions. Within this framework, neuroinflammation emerges as a significant contributing factor. It involves the activation of microglia and astrocytes, leading to the release of pro-inflammatory cytokines and chemokines and the infiltration of peripheral leukocytes into the central nervous system (CNS). These instances result in neuronal damage and neurodegeneration through activated nucleotide-binding domain and leucine-rich repeat containing (NLR) family pyrin domain containing protein 3 (NLRP3) and nuclear factor kappa B (NF-kB) pathways and decreased nuclear factor erythroid 2-related factor 2 (Nrf2) activity. Due to limited effectiveness regarding the inhibition of neuroinflammatory targets using conventional drugs, there is challenging growth in the search for innovative therapies for alleviating neuroinflammation in CNS diseases or even before their onset. Our results indicate that interventions focusing on Interleukin-Driven Immunomodulation, Chemokine (CXC) Receptor Signaling and Expression, Cold Exposure, and Fibrin-Targeted strategies significantly promise to mitigate neuroinflammatory processes. These approaches demonstrate potential anti-neuroinflammatory effects, addressing conditions such as Multiple Sclerosis, Experimental autoimmune encephalomyelitis, Parkinson’s Disease, and Alzheimer’s Disease. While the findings are promising, immunomodulatory therapies often face limitations due to Immune-Related Adverse Events. Therefore, the conduction of randomized clinical trials in this matter is mandatory, and will pave the way for a promising future in the development of new medicines with specific therapeutic targets.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/38259497/
Mesenchymal stem cells-derived extracellular vesicles protect against oxidative stress-induced xenogeneic biological root injury via adaptive regulation of the PI3K/Akt/NRF2 pathway
Xenogeneic extracellular matrices (xECM) for cell support have emerged as a potential strategy for addressing the scarcity of donor matrices for allotransplantation. However, the poor survival rate or failure of xECM-based organ transplantation is due to the negative impacts of high-level oxidative stress and inflammation on seed cell viability and stemness. Herein, we constructed xenogeneic bioengineered tooth roots (bio-roots) and used extracellular vesicles from human adipose-derived mesenchymal stem cells (hASC-EVs) to shield bio-roots from oxidative damage. Pretreatment with hASC-EVs reduced cell apoptosis, reactive oxygen species generation, mitochondrial changes, and DNA damage. Furthermore, hASC-EV treatment improved cell proliferation, antioxidant capacity, and odontogenic and osteogenic differentiation, while significantly suppressing oxidative damage by activating the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and nuclear factor erythroid 2 (NFE2)-related factor 2 (NRF2) nuclear translocation via p62-associated Kelch-like ECH-associated protein 1 (KEAP1) degradation. Inhibition of PI3K/Akt and Nrf2 knockdown reduced antioxidant capacity, indicating that the PI3K/Akt/NRF2 pathway partly mediates these effects. In subcutaneous grafting experiments using Sprague-Dawley rats, hASC-EV administration significantly enhanced the antioxidant effect of the bio-root, improved the regeneration efficiency of periodontal ligament-like tissue, and maximized xenograft function. Conclusively, therefore, hASC-EVs have the potential to be used as an immune modulator and antioxidant for treating oxidative stress-induced bio-root resorption and degradation, which may be utilized for the generation and restoration of other intricate tissues and organs.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/38049845/
Protective effects of oral administration of lactic acid bacteria strains against methylmercury-induced intestinal toxicity in a murine model
The utilization of lactic acid bacteria has been proposed to mitigate the burden of heavy metal exposure through processes probably involving chelation and reduced metal bioaccessibility. We evaluated the effects of daily intake of two strains of lactobacilli (Lactobacillus intestinalis LE1 or Lactobacillus johnsonii LE2) on intestinal toxicity during methylmercury (MeHg) exposure through drinking water (5 mg/L) for two months in mice. MeHg exposure resulted in inflammation and oxidative stress at the colon, as well as an increase in intestinal permeability accompanied by decreased fecal short-chain fatty acids (SCFA). The administration of the strains resulted in a differential protective effect that, based on their chelation capacity, supported the existence of additional mechanisms of action besides chelation. Both strains reduced IL-1β levels and oxidative stress, while LE1 lowered TNF-α, diminished MeHg-induced mucus over-secretion triggered by the IL-4/IL-13/STAT6 pathway, reduced intestinal permeability, and ameliorated inflammation and oxidative stress, probably by acting on the Keap1/Nrf2/ARE pathway. Administration of LE1 partially restored SCFA contents, which could be partly responsible for the positive effects of this strain in alleviating MeHg toxicity. These results demonstrate that lactobacilli strains can be useful tools in reducing the intestinal toxicity of MeHg, the main mercurial form conveyed by food.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/38253281/
Carnitine functions as an enhancer of NRF2 to inhibit osteoclastogenesis via regulating macrophage polarization in osteoporosis
Osteoporosis, which manifests as reduced bone mass and deteriorated bone quality, is common in the elderly population. It is characterized by persistent elevation of macrophage-associated inflammation and active osteoclast bone resorption. Currently, the roles of intracellular metabolism in regulating these processes remain unclear. In this study, we initially performed bioinformatics analysis and observed a significant increase in the proportion of M1 macrophages in bone marrow with aging. Further metabolomics analysis demonstrated a notable reduction in the expression of carnitine metabolites in aged macrophages, while carnitine was not detected in osteoclasts. During the differentiation process, osteoclasts took up carnitine synthesized by macrophages to regulate their own activity. Mechanistically, carnitine enhanced the function of Nrf2 by inhibiting the Keap1-Nrf2 interaction, reducing the proteasome-dependent ubiquitination and degradation of Nrf2. In silico molecular ligand docking analysis of the interaction between carnitine and Keap1 showed that carnitine binds to Keap1 to stabilize Nrf2 and enhance its function. In this study, we found that the decrease in carnitine levels in aging macrophages causes overactivation of osteoclasts, ultimately leading to osteoporosis. A decrease in serum carnitine levels in patients with osteoporosis was found to have good diagnostic and predictive value. Moreover, supplementation with carnitine was shown to be effective in the treatment of osteoporosis.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/38246515/
Aucubin provides protection against cerebral ischaemia-reperfusion injury by suppressing neuronal apoptosis, oxidative stress, and inflammation through the modulation of the AKT-GSK-3β-Nrf2 signal cascade
Aucubin (AU) is a naturally occurring iridoid glycoside known to possess a wide range of pharmacological properties and exhibit a notable protective effect against various pathological conditions. Studies have shown that AU has neuroprotective properties in different neurological diseases. However, its potential protective effects against cerebral ischemia-reperfusion (CIR) injury have not been thoroughly investigated. This study aimed to investigate the impact of AU on CIR injury and explore the underlying mechanism. Cultured neurons treated with AU showed a significant reduction in apoptosis, oxidative stress, and inflammation caused by oxygen-glucose deprivation and reoxygenation (OGD/R). In a rat model of CIR, treatment with AU resulted in a significant decrease in cerebral infarct size and neurological deficits. AU treatment also reversed the increased apoptosis, oxidative stress, and inflammation in the brains of CIR rats. Furthermore, AU was found to enhance the activation of nuclear factor-erythroid 2-related factor 2 (Nrf2), accompanied by increased phosphorylation of serine/threonine-protein kinase AKT and glycogen synthase kinase-3 beta (GSK-3β). The activation of Nrf2 induced by AU was reversed when the AKT-GSK-3β cascade was blocked. Additionally, the neuroprotective effect of AU was significantly reduced when Nrf2 was pharmacologically suppressed. In conclusion, these findings suggest that AU exerts a neuroprotective effect on CIR injury, and this effect is mediated by the activation of Nrf2 through the AKT-GSK-3β axis. This work highlights the potential of AU as a drug candidate for the treatment of CIR injury.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/38246288/
Investigation of the effects of crocin on inflammation, oxidative stress, apoptosis, NF-κB, TLR-4 and Nrf-2/HO-1 pathways in gentamicin-induced nephrotoxicity in rats
The primary limitation of gentamicin (Gm) treatment is its potential to induce nephrotoxicity, which can restrict both its duration and efficacy. This study aims to investigate the protective effects of Crocin (Cr) against Gm-induced nephrotoxicity and its underlying mechanisms, including inflammation, apoptosis, TLR-4, Nrf-2/HO-1 pathways. 36 Sprague Dawley rats were divided into 6 groups for the study. Group I received only saline. Groups II and III were administered 25 and 50mg/kg of crocin, respectively. Group IV was treated with 80mg/kg of Gm. Groups V and VI received 25 and 50mg/kg of crocin, respectively, in addition to Gm administration. Crocin demonstrated protective effects on kidney tissue. It down-regulated the genes NF-κB, COX-2, TLR-4, Bax, and Caspase-3, while up-regulating Bcl-2, Nrf-2, and HO-1. In conclusion, these findings hold promise for the prevention of Gm-induced nephrotoxicity through the modulation of the Nrf-2/HO-1 pathway.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/38246228/
Rebalancing NOX2/Nrf2 to limit inflammation and oxidative stress across gut-brain axis in migraine
Migraine is one of the most common neurological illnesses, and it is characterized by complicated neurobiology. It was confirmed the influence of inflammation and oxidative stress in migraines and also in distal organs such as the intestine. Indeed, the constant bidirectional communication between the Central Nervous System (CNS) and the gastrointestinal (GI) tract, known as the gut-brain axis, has become an attractive target involved in different human disorders. Herein, we explored the role of NADPH oxidase 2 (NOX2) in nitroglycerin (NTG)-induced migraine in mice models to discover the mechanism by which, during migraine attack, oxidative stress is sustained within trigeminal neurons and GI. Considering the inverse relationship between NOX2 and Nrf2, Nrf2 upregulation seems to be a promising approach to decrease NOX2 expression and consequently limit oxidative stress and inflammation spread in neurological and non-neurological diseases. With this aim, we exploited tempol’s Nrf2-inducer ability to better understand the involvement of Nrf2/NOX2 axis in migraine and associated GI comorbidities. Behavioral tests confirmed that tempol, in a dose-dependent manner, moderated clinical signs of migraine and abdominal pain. Moreover, we demonstrated that the decrease in migraine-related symptomatology was strongly linked to the modulation of Nrf2/NOX2 signaling pathway in the brain and colon. In the brain, the rebalancing of Nrf2/NOX2 prevented neuronal loss, decreased glia reactivity while inhibiting NF-κB and NLRP3 inflammasome activation. In the colon, Nrf2 upregulation and consequent NOX2 decrease reduced the histological damage, mast cells infiltration as well as tumor necrosis factor (TNF)-α and interleukin (IL)-1β release. Furthermore, the attenuation of inflammation and oxidative stress led to the restoration of the intestinal barrier through TJs replacement. Taken as a whole, data suggested that the regulation of Nrf2/NOX2 balance is a successful way to reduce neurological and related intestinal impairments during migraine and could be of relevance for migraine-like attacks in humans.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/38244728/
Dextran sodium sulfate (DSS)-induced colitis is alleviated in mice after administration of flavone-derived NRF2-activating molecules
Inflammatory Bowel Disease (IBD) is a chronic and relapsing inflammatory condition characterized by severe symptoms such as diarrhea, fatigue, and weight loss. Growing evidence underscores the direct involvement of the nuclear factor-erythroid 2-related factor 2 (NRF2) in the development and progression of IBD, along with its associated complications, including colorectal cancer. The NRF2 pathway plays a crucial role in cellular responses to oxidative stress, and dysregulation of this pathway has been implicated in IBD. Flavones, a significant subclass of flavonoids, have shown pharmacological impacts in various diseases including IBD, through the NRF2 signaling pathway. In this study, we conducted a screening of compounds with a flavone structure and identified NJK15003 as a promising NRF2 activator. NJK15003 demonstrated potent NRF2 activation, as evidenced by the upregulation of downstream proteins, promoter activation, and NRF2 nuclear translocation in IBD cellular models. Treatment with NJK15003 effectively restored the protein levels of tight junctions in cells treated with dextran sodium sulfate (DSS) and in DSS-treated mice, suggesting its potential to protect cells from barrier integrity disruption in IBD. In DSS-treated mice, the administration of NJK15003 resulted in the prevention of body weight loss, a reduction in colon length shortening, and a decrease in the disease activity index. Furthermore, NJK15003 treatment substantially alleviated inflammatory responses and apoptotic cell death in the colon of DSS-treated mice. Taken together, this study proposes the potential utility of NRF2-activating flavone compounds, exemplified by NJK15003, for the treatment of IBD.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/38242497/
A novel crosstalk between Nrf2 and Smad2/3 bridged by two nuanced Keap1 isoforms with their divergent effects on these distinct family transcription factors
The Keap1-Nrf2 signalling to transcriptionally regulate antioxidant response element (ARE)-driven target genes has been accepted as key redox-sensitive pathway governing a vast variety of cellular stresses during healthy survival and disease development. Herein, we identified two nuanced isoforms α and β of Keap1 in HepG2 cells, arising from its first and another in-frame translation starting codons, respectively. In identifying those differential expression genes monitored by Keap1α and/or Keap1β, an unusual interaction of Keap1 with Smad2/3 was discovered by parsing transcriptome sequencing, Keap1-interacting protein profiling and relevant immunoprecipitation data. Further examination validated that Smad2/3 enable physical interaction with Keap1, as well as its isoforms α and β, by both EDGETSD and DLG motifs in the linker regions between their MH1 and MH2 domains, such that the stability of Smad2/3 and its transcriptional activity are enhanced with their prolonged half-lives and relevant signalling responses from the cytoplasmic to nuclear compartments. The activation of Smad2/3 by Keap1, Keap1α or Keap1β was much likely contributable to a coordinative or another competitive effect of Nrf2, particularly in distinct Keap1-based cellular responses to its cognate growth factor (i.e. TGF-β1) or redox stress (e.g. stimulated by tBHQ and DTT). Overall, this discovery presents a novel functional bridge crossing the Keap1-Nrf2 redox signalling and the TGF-β1-Smad2/3 pathways so as to coordinately regulate the healthy growth and development.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/38242246/
Thiol starvation triggers melanoma state switching in an ATF4 and NRF2-dependent manner
The cystine/glutamate antiporter xCT is an important source of cysteine for cancer cells. Once taken up, cystine is reduced to cysteine and serves as a building block for the synthesis of glutathione, which efficiently protects cells from oxidative damage and prevents ferroptosis. As melanomas are particularly exposed to several sources of oxidative stress, we investigated the biological role of cysteine and glutathione supply by xCT in melanoma. xCT activity was abolished by genetic depletion in the Tyr::CreER; BrafCA; Ptenlox/+ melanoma model and by acute cystine withdrawal in melanoma cell lines. Both interventions profoundly impacted melanoma glutathione levels, but they were surprisingly well tolerated by murine melanomas in vivo and by most human melanoma cell lines in vitro. RNA sequencing of human melanoma cells revealed a strong adaptive upregulation of NRF2 and ATF4 pathways, which orchestrated the compensatory upregulation of genes involved in antioxidant defence and de novo cysteine biosynthesis. In addition, the joint activation of ATF4 and NRF2 triggered a phenotypic switch characterized by a reduction of differentiation genes and induction of pro-invasive features, which was also observed after erastin treatment or the inhibition of glutathione synthesis. NRF2 alone was capable of inducing the phenotypic switch in a transient manner. Together, our data show that cystine or glutathione levels regulate the phenotypic plasticity of melanoma cells by elevating ATF4 and NRF2.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/38219574/
Specific targeting of the NRF2/β-TrCP axis promotes beneficial effects in NASH
Non-alcoholic steatohepatitis (NASH) is a common chronic liver disease that compromises liver function, for which there is not a specifically approved medicine. Recent research has identified transcription factor NRF2 as a potential therapeutic target. However, current NRF2 activators, designed to inhibit its repressor KEAP1, exhibit unwanted side effects. Alternatively, we previously introduced PHAR, a protein-protein interaction inhibitor of NRF2/β-TrCP, which induces a mild NRF2 activation and selectively activates NRF2 in the liver, close to normal physiological levels. Herein, we assessed the effect of PHAR in protection against NASH and its progression to fibrosis. We conducted experiments to demonstrate that PHAR effectively activated NRF2 in hepatocytes, Kupffer cells, and stellate cells. Then, we used the STAM mouse model of NASH, based on partial damage of endocrine pancreas and insulin secretion impairment, followed by a high fat diet. Non-invasive analysis using MRI revealed that PHAR protects against liver fat accumulation. Moreover, PHAR attenuated key markers of NASH progression, including liver steatosis, hepatocellular ballooning, inflammation, and fibrosis. Notably, transcriptomic data indicate that PHAR led to upregulation of 3 anti-fibrotic genes (Plg, Serpina1a, and Bmp7) and downregulation of 6 pro-fibrotic (including Acta2 and Col3a1), 11 extracellular matrix remodeling, and 8 inflammatory genes. Overall, our study suggests that the mild activation of NRF2 via the protein-protein interaction inhibitor PHAR holds promise as a strategy for addressing NASH and its progression to liver fibrosis.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/38184999/


