NRF2 in COST Action 20121
Our quarterly newsletter attempts to provide our latest news and also aims at becoming a forum for analysis of relevant topics on the field of NRF2 and provide comments to some of the most relevant articles published during the quarter. Previous newsletters can be accessed at:
https://benbedphar.org/our-first-newsletter/
https://benbedphar.org/issue-2-abril-2022/
https://benbedphar.org/issue-3-july-2022/
https://benbedphar.org/issue-4-october-2022/
https://benbedphar.org/issue-5-january-2023/
https://benbedphar.org/issue-6-april-2023/
BenBedPhar is now approaching the equator of its duration, and many exciting activities have been done during these two years. One of them is the introductory video that can be seen in our webpage:
https://benbedphar.org/about-benbedphar/
Our grants program is aimed at developing strong visibility and scientific interactions around the NRF2 community. So far, we have funded nine Short-Term Scientific Missions, three grants for members of Inclusiveness Target Countries, and five Dissemination Conference grants. These activities are essential to developing strong scientific interactions among EU teams. Due novel collaborations provided by the BenBedPhar umbrella, many members have published together over 30 articles and submitted many collaborative grant applications to international and local funding bodies. These activities will increase during the next year with additional articles, where we will provide information about high quality tools, drugs, standard procedures, biobanks, animal models of chronic diseases and biomarkers, in the field of NRF2.
On June 26-30, we organized our first training school in Smolenice castle, near Bratislava (Slovakia). It was a fantastic experience that was effectively organized by Iveta Bernatova and her colleagues. Seven BenBedPhar speakers presented state of the art concepts on the participation of NRF2 in several chronic diseases, oxidative stress, and ageing. One interesting activity was the poster presentation by over 25 trainees. The lectures can be downloaded at:
https://benbedphar.org/training-school-nrf2-in-noncommunicable-diseases-from-bench-to-bedside/
On July 12th, we had one more edition of our Redox Webinar Series in collaboration with Sapienza University of Rome. This time the topic was “NRF2 and Neuroinflammation” and we enjoyed the participation of three outstanding speakers: Ana Rojo and Isabel Lastres Beker from Autonomous University of Madrid (Spain), and Valeria Cordone from the University of Ferrara (Italy). See more details at:
https://benbedphar.org/webinar-nrf2-and-neuroinflammation/
On September 7th, we had the 2023 BenBedPhar Open day, with speakers from five EU institutions in Spain (Antonio Cuadrado), Germany (Andreas Daiber), Romania (Gina Manda), United Kingdom (Laureano de la Vega), and Portugal (Sandra Tenreiro). We used a hybrid format, comprising the audience in five conference rooms and online. It was quite an exciting and successful experience nicely handled by our WG5 members Isabel Lastres and Ana Rita Carlos. We had 90 in person and 55 online participants. See more details at:
https://benbedphar.org/open-days-2/
On October 12-13 we had our Management Committee meeting and our scientific meeting in the wonderful city of Graz, Austria. We were honored by the participation of Prof. Masayuki Yamamoto, who gave the plenary lecture. The local organizers, Brigitte Winklhofer-Roob and Christina Morgenstern, prepared an excellent scientific program that can be seen at:
https://benbedphar.org/5th-benbedphar-scientific-meeting/
At the MC meeting of Graz, mentioned above, we presented a portfolio of new activities to be carried out during the next year, including two scientific meetings, two training schools and a program of STSM, ITC and DC grants. These new activities will help BenBedPhar to go to the next step, which is the reinforcement of clinical and economical approaches towards NRF2-related therapies.
Antonio Cuadrado
Chair of COST Action 20121, BenBedPhar
Autonomous University of Madrid
Comments from the Working Groups
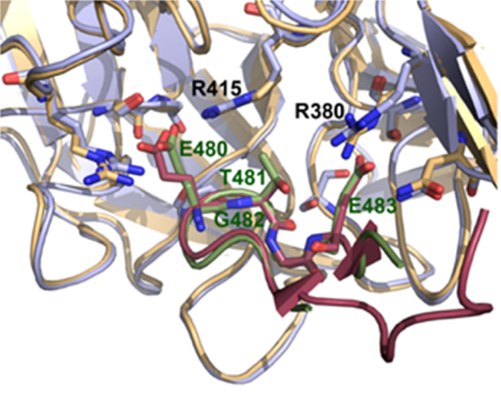
Dipeptidyl peptidase 3 is involved in cancer development through the activation of NRF2-KEAP1 signalling pathway
ETGE-containing protein dipeptidyl peptidase 3 (DPP3) has been identified as one of the competitive interactors of KEAP1 whose overexpression activates NRF2-driven transcription (Hast et al. 2013). KEAP1-DPP3 interaction is induced by oxidative stress and DPP3 overexpression in breast cancer correlates with increased expression of NRF2-controlled genes and poor prognosis (Lu et al. 2017). DPP3 binds KEAP1 through the ETGE motif located on the unstructured loop. Crystal structure of the complex between Kelch domain of KEAP1 and 11 amino acids DPP3 ETGE peptide shows that DPP3 binds Kelch domain in a similar manner as NRF2 (Figure 1). Molecular simulations indicate that ETGE loop in DPP3 is held to the protein body by hydrogen bonds and prior to binding to Kelch domain of KEAP1, the loop has to detach from the protein body (Matić et al. 2021). The combined experimental and computational study of several DPP3 mutants whose genomic sequences were found in cancer showed that R623W mutation in one of the amino acids that keep ETGE loop attached to the DPP3 protein body lowers the work needed to detach the ETGE loop, and consequentially, increases the affinity of the mutated protein towards Kelch domain, while overexpression of DPP3-R623W causes the upregulation of the expression of NQO1 mRNA and protein (Matić et al. 2022). These results indicate that DPP3 could cause the activation of NRF2 in cancer through the overexpression of wild type protein, but also through the expression of mutants with higher affinity towards Kelch domain.
References:
Hast, B.E., D. Goldfarb, K.M. Mulvaney, M.A. Hast, P.F. Siesser, F. Yan, D.N. Hayes, and M.B. Major. 2013. Proteomic analysis of ubiquitin ligase KEAP1 reveals associated proteins that inhibit NRF2 ubiquitination. Cancer Res. 73:2199–2210.
Lu, K., A.L. Alcivar, J. Ma, T.K. Foo, S. Zywea, Y. Huo, T.W. Kensler, M.L. Gatza, and B. Xia. 2017. DPP3 in NRF2 Signaling and Breast Cancer. Free Radic. Biol. Med. 100:S132.
Matic, S., I. Kekez, M. Tomin, F. Bogar, F. Supljika, S. Kazazic, M. Hanic, S. Jha, H. Brkic, B. Bourgeois, T. Madl, K. Gruber, P. Macheroux, D. Matkovic-Calogovic, M. Matovina, and S. Tomic. 2021. Binding of dipeptidyl peptidase III to the oxidative stress cell sensor Kelch-like ECH-associated protein 1 is a two-step process. J. Biomol. Struct. & Dyn. 39:6870–6881.
Matić, S., A. Tomašić Paić, S. Sobočanec, M. Pinterić, G. Pipalović, M. Martinčić, M. Matovina, and S. Tomić. 2022. Interdisciplinary Study of the Effects of Dipeptidyl-Peptidase III Cancer Mutations on the KEAP1-NRF2 Signaling Pathway. Int. J. Mol. Sci. 23:1994.
Mihaela Matovina
WG1 member
Ruđer Bošković Institute
Division of Organic Chemistry and Biochemistry
Laboratory of Protein Biochemistry and Molecular Modelling
NRF2 regulates glucose uptake and metabolism in neurons and astrocytes
The transcription factor Nrf2 and its repressor Keap1 mediate cell stress adaptation by inducing expression of genes regulating cellular detoxification, antioxidant defence and energy metabolism. Energy production and antioxidant defence employ NADH and NADPH respectively as essential metabolic cofactors; both are generated in distinct pathways of glucose metabolism, and both pathways are enhanced by Nrf2 activation. Here, we examined the role of Nrf2 on glucose distribution and the interrelation between NADH production in energy metabolism and NADPH homeostasis using glio-neuronal cultures isolated from wild-type, Nrf2-knockout and Keap1-knockdown mice. Employing advanced microscopy imaging of single live cells, including multiphoton fluorescence lifetime imaging microscopy (FLIM) to discriminate between NADH and NADPH, we found that Nrf2 activation increases glucose uptake into neurons and astrocytes. Glucose consumption is prioritized in brain cells for mitochondrial NADH and energy production, with a smaller contribution to NADPH synthesis in the pentose phosphate pathway for redox reactions. As Nrf2 is suppressed during neuronal development, this strategy leaves neurons reliant on astrocytic Nrf2 to maintain redox balance and energy homeostasis.
Adapted from:
Esteras N, Blacker TS, Zherebtsov EA, Stelmashuk OA, Zhang Y, Wigley WC, Duchen MR, Dinkova-Kostova AT, Abramov AY. Nrf2 regulates glucose uptake and metabolism in neurons and astrocytes. Redox Biol. 2023 Jun;62:102672. doi: 10.1016/j.redox.2023.102672. Epub 2023 Mar 14. PMID: 36940606; PMCID: PMC10034142.
Albena Kinkova-Kostova
WG2 leader
University of Dundee, UK
Genetic variability in NRF2-KEAP1 axis is associated with Alzheimer’s disease and cognitive impairment
Oxidative stress and inflammation are important processes contributing to the pathogenesis in Alzheimer’s disease (AD). Numerous risk factors, including complex genetic background, can reduce the antioxidative potential of the brain and can also affect typical AD hallmarks: amyloid plaques and neurofibrillary tangles. Complex interplay between those mechanisms are similarly important in mild cognitive impairment (MCI), a pre-dementia stage of the disease. NRF2 (encoded by NFE2L2) and KEAP1 are two of the main regulators of redox balance and inflammation, crucial for stress response. Although their potential in the neurodegeneration is extensively studied, the effect of genetic variability in NFE2L2 and KEAP1 remains unclear. Our aim was to evaluate the association of common polymorphisms in NFE2L2 and KEAP1 with cerebrospinal fluid (CSF) biomarkers and cognitive impairment in patients with MCI and AD.
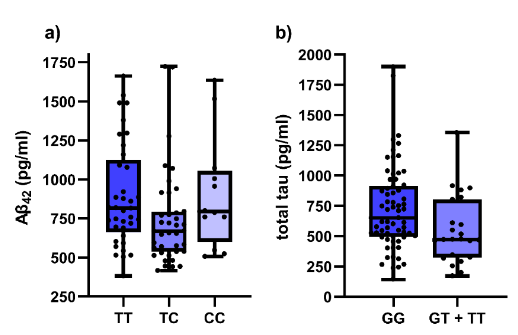
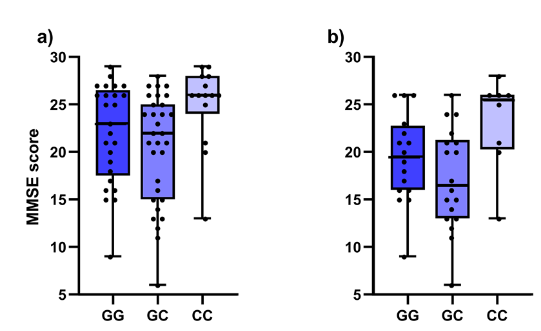
Our study included 54 AD patients, 14 MCI patients with pathological CSF biomarker levels and 20 MCI patients with normal CSF biomarker levels. Patients were diagnosed according to clinical criteria. Levels of Aβ1-42, Aβ42/40, total tau and p-tau181 were measured in CSF. Considering locally validated cut-off levels, decreased Aβ1-42, Aβ42/40 and elevated total tau and p-tau181 are features of AD. The Mini-Mental State Exam (MMSE) was used for the assessment of cognitive impairment. DNA was isolated from blood samples and all patients were genotyped for three NFE2L2 (rs6706649, rs6721961, rs35652124) and two KEAP1 (rs1048290, rs9676881) polymorphisms using competitive allele-specific PCR. Association of polymorphisms with CSF biomarker levels and MMSE was evaluated using nonparametric tests.
In the entire cohort, carriers of at least one polymorphic NFE2L2 rs35652124 allele had lower CSF Aβ1-42 levels (p = 0.031). Additionally, decreased total tau was observed in carriers of at least one polymorphic T allele in NFE2L2 rs6721961 (p = 0.020). Significant associations with MMSE cognitive decline score were observed for both genes. Carriers of two polymorphic alleles in KEAP1 rs1048290 and rs9676881 (both p = 0.019) scored higher on MMSE. Both associations remained significant if only AD patients were included in the analysis: carriers of two polymorphic KEAP1 rs1048290 and rs9676881 alleles scored higher on MMSE (both p = 0.035). On the other hand, polymorphic NFE2L2 rs35652124 C allele was associated with lower test scores in the entire cohort in both additive (p = 0.030) and dominant model (p = 0.024), but not in the AD cohort.
Multiple effects of NFE2L2 and KEAP1 on dementia and AD were found in our study. Polymorphisms in NRF2-KEAP1 axis were associated with CSF AD biomarkers and cognitive test score in Slovenian patients with AD or MCI (Figures 1 and 2). Our data supports the potentially crucial role of NRF2-KEAP1 axis in neurodegeneration and could partially explain the missing connection between oxidative stress and neurodegeneration.
Reference:
Vogrinc D, Gregorič Kramberger M, Emeršič A, Čučnik S, Goričar K, Dolžan V. Genetic Polymorphisms in Oxidative Stress and Inflammatory Pathways as Potential Biomarkers in Alzheimer’s Disease and Dementia. Antioxidants 2023;12(2):316.
David Vogrinc, Katja Goričar, Vita Dolžan
WG3 members
Pharmacogenetics Laboratory, Institute of Biochemistry and Molecular Genetics, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
Reata Pharmaceuticals and its Asia partner Kyowa Kirin announced the discontinuation of the clinical development of its NTF2 inducers program in kidney disease
Masashi Miyamoto, the CEO of Kyowa Kirin Co. announced at May 10 of 2023 the discontinuation of the clinical development program for bardoxolone methyl (RTA 402) a popular Nrf2 activator and small-molecule compound licensed from Reata Pharmaceuticals.
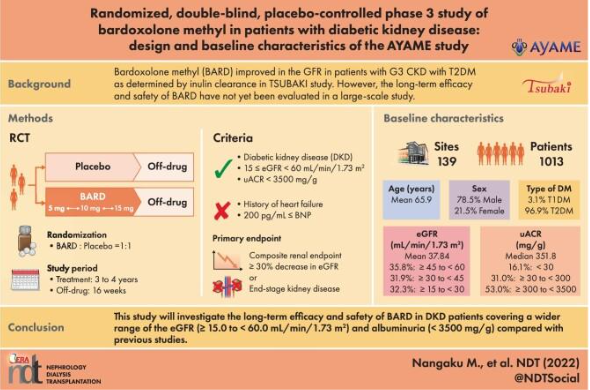
Kyowa Kirin has started a Phase III clinical study in Japan of RTA 402, which has been designated for the treatment of diabetic kidney disease by the Ministry of Health, Labor and Welfare of Japan. This multi-center, randomized, double-blind, placebo-controlled study is designed to assess the efficacy and safety of RTA 402 for diabetic kidney disease (1).
The Pharmaceuticals and Medical Devices Agency (PMDA) of Japan has pointed out that not only the primary and the key secondary endpoints, but also the result regarding the secondary endpoints “Time to onset end-stage renal disease (ESRD)” and others should be discussed for submitting a marketing authorization application of RTA 402 for diabetic kidney disease.
Primary endpoint: “Time to onset a ≥ 30% decrease in eGFR* from baseline or ESRD”
Secondary endpoint: “Time to onset a ≥ 40% decrease in eGFR from baseline or ESRD”
Other secondary endpoints: “Time to onset a ≥ 53% decrease in eGFR from baseline or ESRD”
“Time to onset ESRD” and “Change in eGFR from baseline at each evaluation time point”.
It was expecting positive results from a Phase III study by a Japanese licensee for diabetic kidney disease after a US rejection for Alport Syndrome last year. It was found no separation in ESRD events between bardoxolone and placebo groups, and US company Reata and Asia partner Kyowa Kirin have now decided to end development in CKD indications after a missed secondary endpoint seen as critical to approval in JapanAdditionally, based on the results of AYAME and its potential regulatory impact, the promoters will withdraw their bardoxolone CKD programs, including the FALCON study, a Phase 3, international, multicenter, randomized, double-blind, placebo-controlled trial studying the safety and efficacy of bardoxolone in patients with autosomal dominant polycystic kidney disease (ADPKD) and EAGLE clinical trials an open-label extension study in patients with Alport syndrome and ADPKD, despite of the promising results of bardoxolone methyl in previous clinical trial (2).
After the tremendous economic effort made in the last years including the previous breakdown in 2013 in BEAM study (3), it is very disappointing to see how it halts the development of this promising research program in kidney disease. Chronic renal insufficiency (CKD) is a pathology that presents epidemic characteristics and produces devastating complications for the patient and his or her environment, which it is currently considered a public health problem. Nrf2 activators, in this case bardoxolone methyl, have been for the last years one of the most promising treatments for kidney disease and this decision leaves patients orphaned without effective treatment to prevent inflammation and renal dysfunction. Thus, new approaches to treatment are required urgently to prevent the dramatic consequences of CKD in the patients’s lives.
References:
1. Nangaku M, Takama H, Ichikawa T, Mukai K, Kojima M, Suzuki Y, et al. Randomized, double-blind, placebo-controlled phase 3 study of bardoxolone methyl in patients with diabetic kidney disease: design and baseline characteristics of the AYAME study. Nephrol Dial Transplant. 2023;38(5):1204-16.
2. Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. The New England journal of medicine. 2011;365(4):327-36.
3. Zoja C, Corna D, Nava V, Locatelli M, Abbate M, Gaspari F, et al. Analogs of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. Am J Physiol Renal Physiol. 2013;304(6):F808-19.
Santiago Cuevas
WG4 leader
BioMedical Research Institute of Murcia (IMIB), Murcia, Spain
The Protective Role of NRF2 Pathway in the Antiphospholipid Antibody Syndrome
Oxidative stress is considered a key element that largely contributes to Antiphospholipid Antibody Syndrome (APS) pathogenesis, a systemic autoimmune disease characterized by arterial and venous thrombosis and/or pregnancy morbidity associated with circulating “anti-phospholipid antibodies” (aPLs), such as lupus anticoagulant, anticardiolipin antibodies and anti-β2-glycoprotein I antibodies. The chronic oxidative stress and the dysregulation of the immune system leads to the loss of tolerance, which drives autoantibody production and inflammation with the development of endothelial dysfunction. A variety of molecular pathways become activated, including those resulting in an overproduction of reactive oxygen species (ROS), inflammatory signaling and apoptotic cell death. Among the “survival” signaling factors, the transcription factor Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) contributes to anti-inflammatory and antioxidant processes and thereby prevents cell death by upregulating various cytoprotective enzymes and proteins.
In recent review published in the Biomolecules highlights the role of oxidative stress as a regulatory checkpoint in antiphospholipid autoantibody production. The authors discussed the available evidence for the involvement of aPLs in the induction of a pro-oxidative state in APS patients and the possible use of oxidative stress biomarkers in the patient management (Figure 1). The pathogenic roles of oxidative stress in the post-translational modifications of antigens associated with APS has been discussed and a connection between the environmental factors and oxidative stress and inflammation highlighted. Moreover, the importance of oxidative stress in the activation of multiple signaling pathways that accelerate the progression and exacerbation of the symptoms of APS has been examined and the protective role of food supplements and NRF2 activators in APS patients has been elucidated. This knowledge provides new insights into the pathogenesis of APS and introduces new hypotheses for valuable therapeutic targets, including personalized redox nutraceutical medicine approaches (Figure 1). However, far more studies are warranted to fully understand the role of the NRF2 in APS patients, especially to determine the level of NRF2 activation that would significantly slow disease progression when various NRF2 activators are used. In silico studies, cellular and animal models should be established to test the efficacy and side effects of NRF2 activators.
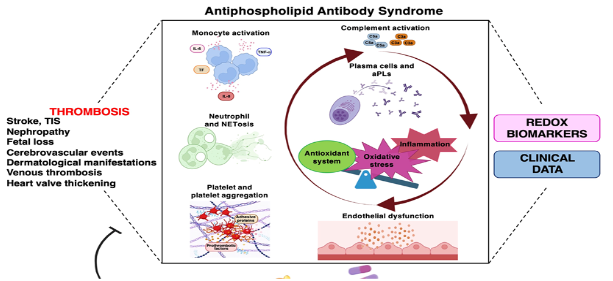
References:
Sorice M, Profumo E, Capozzi A, Recalchi S, Riitano G, Di Veroli B, Saso L, Buttari B. Oxidative Stress as a Regulatory Checkpoint in the Production of Antiphospholipid Autoantibodies: The Protective Role of NRF2 Pathway. Biomolecules. 2023 Aug 5;13(8):1221. doi: 10.3390/biom13081221. PMID: 37627286; PMCID: PMC10452087.
Brigitta Buttari
WG5 Leader
Istituto Superiore di Sanità, Itali
The role of acrolein for E‑cigarette vapour condensate mediated activation of NADPH oxidase in cultured endothelial cells and macrophages
Electronic cigarettes (E-cigarettes) have recently become a popular alternative to traditional tobacco cigarettes. Despite being marketed as a healthier alternative, increasing evidence shows that E-cigarette vapour could cause adverse health effects. It has been postulated that degradation products of E-cigarette liquid, mainly reactive aldehydes, are responsible for those
effects. Previously, we have demonstrated that E-cigarette vapour exposure causes oxidative stress, inflammation, apoptosis, endothelial dysfunction and hypertension by activating NADPH oxidase in a mouse model. To better understand oxidative stress mechanisms, we have exposed cultured endothelial cells and macrophages to condensed E-cigarette vapour (E-cigarette condensate) and acrolein. In both endothelial cells (EA.hy 926) and macrophages (RAW 264.7), we have observed that E-cigarette condensate incubation causes cell death. Since recent studies have shown that among toxic aldehydes found in cigarette vapour, acrolein plays a prominent role, we have incubated the same cell lines with increasing concentrations of acrolein. Upon incubation with acrolein, a translocation of Rac1 to the plasma membrane has been observed, accompanied by an increase in oxidative stress. Whereas reactive oxygen species (ROS) formation by acrolein in cultured endothelial cells was mainly intracellular, the release of ROS in cultured macrophages was both intra- and extracellular. Our data also demonstrate that acrolein activates the nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant pathway and, in general, could mediate E-cigarette vapour-induced oxidative stress and cell death (Figure 1). More mechanistic insight is needed to clarify the toxicity associated with E-cigarette consumption and the possible adverse effects on human health.
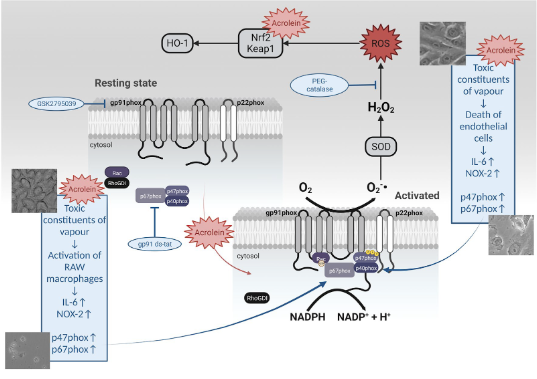
Adapted from:
Kuntic I, Kuntic M, Oelze M, Stamm P, Karpi A, Kleinert H, Hahad O, Münzel T, Daiber A. The role of acrolein for E-cigarette vapour condensate mediated activation of NADPH oxidase in cultured endothelial cells and macrophages. Pflugers Arch. 2023;475(7):807-821.
Andreas Daiber, WG2,3,5
University Medical Center of the Johannes Gutenberg
University Mainz, Germany
Hot from Pubmed
A systems approach reveals species differences in hepatic stress response capacity
To minimize the occurrence of unexpected toxicities in early phase preclinical studies of new drugs, it is vital to understand fundamental similarities and differences between preclinical species and humans. Species differences in sensitivity to acetaminophen (APAP) liver injury have been related to differences in the fraction of the drug that is bioactivated to the reactive metabolite N-acetyl-p-benzoquinoneimine (NAPQI). The authors used physiologically-based pharmacokinetic modeling to identify oral doses of APAP (300 and 1000 mg/kg in mice and rats, respectively) yielding similar hepatic burdens of NAPQI to enable the comparison of temporal liver tissue responses under conditions of equivalent chemical insult. Despite pharmacokinetic and biochemical verification of the equivalent NAPQI insult, serum biomarker and tissue histopathology analyses revealed that mice still exhibited a greater degree of liver injury than rats. Transcriptomic and proteomic analyses highlighted the stronger activation of stress response pathways (including the Nrf2 oxidative stress response and autophagy) in the livers of rats, indicative of a more robust transcriptional adaptation to the equivalent insult. Components of these pathways were also found to be expressed at a higher basal level in the livers of rats compared with both mice and humans. Our findings exemplify a systems approach to understanding differential species sensitivity to hepatotoxicity. Multi-omics analysis indicated that rats possess a greater basal and adaptive capacity for hepatic stress responses than mice and humans, with important implications for species selection and human translation in the safety testing of new drug candidates associated with reactive metabolite formation.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37647630/
Metabolic reprogramming in Nrf2-driven proliferation of normal rat hepatocytes
Cancer cells reprogram their metabolic pathways to support bioenergetic and biosynthetic needs and to maintain their redox balance. In several human tumors the Keap1-Nrf2 system controls proliferation and metabolic reprogramming by regulating the pentose phosphate pathway (PPP). However, whether this metabolic reprogramming also occurs in normal proliferating cells is unclear. To define the metabolic phenotype in normal proliferating hepatocytes, the authors induced cell proliferation in the liver by three distinct stimuli: liver regeneration by partial hepatectomy (PH) and hepatic hyperplasia induced by two direct mitogens, lead nitrate (LN) or triiodothyronine (T3). Following LN treatment, well-established features of cancer metabolic reprogramming including enhanced glycolysis, oxidative PPP, nucleic acid synthesis, NAD+/NADH synthesis and altered amino acid content as well as downregulated oxidative phosphorylation (OXPHOS) occurred in normal proliferating hepatocytes displaying Nrf2 activation. Genetic deletion of Nrf2 blunted LN-induced PPP activation and suppressed hepatocyte proliferation. Moreover, Nrf2 activation and following metabolic reprogramming did not occur when hepatocyte proliferation was induced by PH or T3. Many metabolic changes in cancer cells are shared by proliferating normal hepatocytes in response to a hostile environment. Nrf2 activation is essential for bridging metabolic changes with crucial components of cancer metabolic reprogramming including the activation of oxidative PPP. This study demonstrates that matured hepatocytes exposed to LN undergo a cancer-like metabolic reprogramming and offers a rapid and useful in vivo model to study the molecular alterations underpinning the differences/similarities of metabolic changes in normal and neoplastic hepatocytes.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37603610/
miR-200a attenuated oxidative stress, inflammation, and apoptosis in dextran sulfate sodium-induced colitis through activation of Nrf2
Oxidative stress and inflammatory responses are critical factors in ulcerative colitis disease pathogenesis. Nuclear factor erythroid 2-related factor 2 (Nrf2) modulates oxidative stress and suppresses inflammatory responses, and the protective benefits of Nrf2 activation have been associated with the therapy of ulcerative colitis. MicroRNA-200a (miR-200a) could target Kelch-like ECH-associated protein 1 (Keap1) and activate the Nrf2-regulated antioxidant pathway. Nevertheless, whether miR-200a modulates the Keap1/Nrf2 pathway in dextran sulfate sodium (DSS)-induced colonic damage is unknown. Here,this research intends to examine the impact of miR-200a in the model of DSS-induced colitis. Prior to DSS intervention, miR-200a was overexpressed in mice for four weeks using an adeno-associated viral (AAV) vector to address this problem. ELISA detected the concentration of inflammation-related cytokines. The genes involved in inflammatory reactions and oxidative stress were identified using quantitative reverse transcription-polymerase chain reaction (qRT-PCR), western blot, and immunofluorescence. Moreover, it was applied siRNAs to weakened Nrf2 expression to confirm the hypothesis that miR-200a provided protection via Nrf2. The present study discovered miR-200a down-regulation, excessive inflammatory activation, enterocyte apoptosis, colonic dysfunction, and Keap1/Nrf2 antioxidant pathway inactivation in mouse colitis and NCM460 cells under DSS induction. However, these data demonstrated that miR-200a overexpression represses Keap1 and activates the Nrf2 antioxidant pathway, thereby alleviating these adverse alterations in animal and cellular models. Significantly, following Nrf2 deficiency, the authors failed to observe the protective benefits of miR-200a against colonic damage. Taken together, through activating the Keap1/Nrf2 signaling pathway, miR-200a protected against DSS-induced colonic damage. These studies offer an innovative therapeutic approach for ulcerative colitis.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37646040/
A NRF2-induced secretory phenotype activates immune surveillance to remove irreparably damaged cells
While it is well established that the KEAP1-NRF2 pathway regulates the main inducible cellular response to oxidative stress, this cytoprotective function of NRF2 could become deleterious to the host if it confers survival onto irreparably damaged cells. In this regard, the authors have found that in diseased states, NRF2 promotes the transcriptional activation of a specific subset of the senescence-associated secretory phenotype (SASP) gene program, which was have named the NRF2-induced secretory phenotype (NISP). In two models of hepatic disease using Pten::Keap1 and Keap1::Atg7 double knockout mice, the authors found that the NISP functions in the liver to recruit CCR2 expressing monocytes, which function as immune system effector cells to directly remove the damaged cells. Through activation of this immune surveillance pathway, in non-transformed cells, NRF2 functions as a tumour suppressor to mitigate the long-term survival of damaged cells which otherwise would be detrimental for host survival. This pathway represents the final stage of the oxidative stress response, as it allows cells to be safely removed if the macromolecular damage caused by the original stressor is so extensive that it is beyond the repair capacity of the cell.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37597423/
The NRF2-p97-NRF2 negative feedback loop
p97 is a ubiquitin-targeted ATP-dependent segregase that regulates proteostasis, in addition to a variety of other cellular functions. Previously, the authors demonstrated that p97 negatively regulates NRF2 by extracting ubiquitylated NRF2 from the KEAP1-CUL3-RBX1 E3 ubiquitin ligase complex, facilitating proteasomal destruction. In the current study, it was identified p97 as an NRF2-target gene that contains a functional ARE, indicating the presence of an NRF2-p97-NRF2 negative feedback loop that maintains redox homeostasis. Using CRISPR/Cas9 genome editing, it was generated endogenous p97 ARE-mutated BEAS-2B cell lines. These p97 ARE-mutated cell lines exhibit altered expression of p97 and NRF2, as well as a compromised response to NRF2 inducers. Importantly, it was also found a positive correlation between NRF2 activation and p97 expression in human cancer patients. Finally, using chronic arsenic-transformed cell lines, the authors demonstrated a synergistic effect of NRF2 and p97 inhibition in killing cancer cells with high NRF2 and p97 expression. This study suggests dual upregulation of NRF2 and p97 occurs in certain types of cancers, suggesting that inhibition of both NRF2 and p97 could be a promising treatment strategy for stratified cancer patients.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37573837/
Limited expression of Nrf2 in neurons across the central nervous system
Nrf2, encoded by the gene Nfe2l2, is a broadly expressed transcription factor that regulates gene expression in response to reactive oxygen species (ROS) and oxidative stress. It is commonly referred to as a ubiquitous pathway, but this generalization overlooks work indicating that Nrf2 is essentially unexpressed in some neuronal populations. To explore whether this pattern extends throughout the central nervous system (CNS), the authors quantified Nfe2l2 expression and chromatin accessibility at the Nfe2l2 locus across multiple single cell datasets. In both the mouse and human CNS, Nfe2l2 was repressed in almost all mature neurons, but highly expressed in non-neuronal support cells, and this pattern was robust across multiple human CNS diseases. A subset of key Nrf2 target genes, like Slc7a11, also remained low in neurons. Thus, these data suggest that while most cells express Nfe2l2, with activity determined by ROS levels, neurons actively avoid Nrf2 activity by keeping Nfe2l2 expression low.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37544245/
Transcriptomic-based evaluation of trichloroethylene glutathione and cysteine conjugates demonstrate phenotype-dependent stress responses in a panel of human in vitro models
Environmental or occupational exposure of humans to trichloroethylene (TCE) has been associated with different extrahepatic toxic effects, including nephrotoxicity and neurotoxicity. Bioactivation of TCE via the glutathione (GSH) conjugation pathway has been proposed as underlying mechanism, although only few mechanistic studies have used cell models of human origin. In this study, six human derived cell models were evaluated as in vitro models representing potential target tissues of TCE-conjugates: RPTEC/TERT1 (kidney), HepaRG (liver), HUVEC/TERT2 (vascular endothelial), LUHMES (neuronal, dopaminergic), human induced pluripotent stem cells (hiPSC) derived peripheral neurons (UKN5) and hiPSC-derived differentiated brain cortical cultures containing all subtypes of neurons and astrocytes (BCC42). A high throughput transcriptomic screening, utilizing mRNA templated oligo-sequencing (TempO-Seq), was used to study transcriptomic effects after exposure to TCE-conjugates. Cells were exposed to a wide range of concentrations of S-(1,2-trans-dichlorovinyl)glutathione (1,2-DCVG), S-(1,2-trans-dichlorovinyl)-L-cysteine (1,2-DCVC), S-(2,2-dichlorovinyl)glutathione (2,2-DCVG), and S-(2,2-dichlorovinyl)-L-cysteine (2,2-DCVC). 1,2-DCVC caused stress responses belonging to the Nrf2 pathway and Unfolded protein response in all the tested models but to different extents. The renal model was the most sensitive model to both 1,2-DCVC and 1,2-DCVG, with an early Nrf2-response at 3 µM and hundreds of differentially expressed genes at higher concentrations. Exposure to 2,2-DCVG and 2,2-DCVC also resulted in the upregulation of Nrf2 pathway genes in RPTEC/TERT1 although at higher concentrations. Of the three neuronal models, both the LUHMES and BCC42 showed significant Nrf2-responses and at higher concentration UPR-responses, supporting recent hypotheses that 1,2-DCVC may be involved in neurotoxic effects of TCE. The cell models with the highest expression of γ-glutamyltransferase (GGT) enzymes, showed cellular responses to both 1,2-DCVG and 1,2-DCVC. Little to no effects were found in the neuronal models from 1,2-DCVG exposure due to their low GGT-expression. This study expands the knowledge on tissue specificity of TCE S-conjugates and emphasizes the value of human cell models together with transcriptomics for such mechanistic studies.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/36576512/
Nrf2 Signaling Pathway: a Potential Therapeutic Target in Combating Oxidative Stress and Neurotoxicity in Chemotherapy-Induced Cognitive Impairment
Chemotherapy-induced cognitive impairment (CICI) is one of the major adverse effects of antineoplastic drugs, which decrease the quality of life in cancer survivors. Extensive experimental and clinical research suggests that chemotherapeutic drugs generate an enormous amount of reactive oxygen species (ROS), contributing to oxidative stress, neuroinflammation, blood-brain barrier (BBB) disruption, and neuronal death, eventually leading to CICI. Despite the progress in exploring different pathological mechanisms of CICI, effective treatment to prevent CICI progression has not been developed yet. Nrf2 is the principal transcription factor that regulates cellular redox balance and inflammation-related gene expression. Emerging evidence suggests that upregulation of Nrf2 and its target genes could suppress oxidative stress, and neuroinflammation, restore BBB integrity, and increase neurogenesis. This review discusses the role of Nrf2 in CICI, how it responds to oxidative stress, inflammation, neurotoxicity, and potential Nrf2 activators that could be used to enhance Nrf2 activation in CICI.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37644279/
Asparagine restriction enhances CD8+ T cell metabolic fitness and antitumoral functionality through an NRF2-dependent stress response
Robust and effective T cell immune surveillance and cancer immunotherapy require proper allocation of metabolic resources to sustain energetically costly processes, including growth and cytokine production. Here, the authors show that asparagine (Asn) restriction on CD8+ T cells exerted opposing effects during activation (early phase) and differentiation (late phase) following T cell activation. Asn restriction suppressed activation and cell cycle entry in the early phase while rapidly engaging the nuclear factor erythroid 2-related factor 2 (NRF2)-dependent stress response, conferring robust proliferation and effector function on CD8+ T cells during differentiation. Mechanistically, NRF2 activation in CD8+ T cells conferred by Asn restriction rewired the metabolic program by reducing the overall glucose and glutamine consumption but increasing intracellular nucleotides to promote proliferation. Accordingly, Asn restriction or NRF2 activation potentiated the T cell-mediated antitumoral response in preclinical animal models, suggesting that Asn restriction is a promising and clinically relevant strategy to enhance cancer immunotherapy. This study revealed Asn as a critical metabolic node in directing the stress signaling to shape T cell metabolic fitness and effector functions.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37550596/
Alpha-Lipoic Acid Ameliorates Doxorubicin-Induced Cognitive Impairments by Modulating Neuroinflammation and Oxidative Stress via NRF-2/HO-1 Signaling Pathway in the Rat Hippocampus
Chemotherapy-induced cognitive impairment (CICI) is a common complication associated with the use of chemotherapeutics. Doxorubicin (DOX) is a reactive oxygen species (ROS) producing anticancer agent capable of causing potential neurotoxic effects via cytokine-induced oxidative and nitrosative damage to brain tissues. On the other hand, alpha-lipoic acid (ALA), a nutritional supplement, is reputable for its excellent antioxidant, anti-inflammatory, and anti-apoptotic activities. Consequently, the objective of the current investigation was to examine any potential neuroprotective and memory-improving benefits of ALA against DOX-induced behavioral and neurological anomalies. DOX (2 mg/kg/week, i.p.) was administrated for 4 weeks to Sprague-Dawley rats. ALA (50, 100, and 200 mg/kg) was administered for 4 weeks. The Morris water maze (MWM) and novel objective recognition task (NORT) tests were used to assess memory function. Biochemical assays with UV-visible spectrophotometry were used to analyze oxidative stress markers [malondialdehyde (MDA), protein carbonylation (PCO)], endogenous antioxidants [reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px)] and acetylcholinesterase (AChE) activity in hippocampal tissue. Inflammatory markers [tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and nuclear factor kappa B (NF-κB)], nuclear factor erythroid 2-related factor-2 (NRF-2) and hemeoxygenase-1 (HO-1) levels were estimated using enzyme-linked immunosorbent assay (ELISA). In addition, reactive oxygen species (ROS) levels were measured in hippocampus tissue using 2-7-dichlorofluorescein-diacetate (DCFH-DA) assay with fluorimetry. ALA treatment significantly protected against DOX-induced memory impairment. Furthermore, ALA restored hippocampal antioxidants, halted DOX-induced oxidative and inflammatory insults via upregulation of NRF-2/HO-1 levels, and alleviated the increase in NF-κB expression. These results indicate that ALA offers neuroprotection against DOX-induced cognitive impairment, which could be attributed to its antioxidant potential via the NRF-2/HO-1 signaling pathway.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37017891/
CCN2 Activates RIPK3, NLRP3 Inflammasome, and NRF2/Oxidative Pathways Linked to Kidney Inflammation
Inflammation is a key characteristic of both acute and chronic kidney diseases. Preclinical data suggest the involvement of the NLRP3/Inflammasome, receptor-interacting protein kinase-3 (RIPK3), and NRF2/oxidative pathways in the regulation of kidney inflammation. Cellular communication network factor 2 (CCN2, also called CTGF in the past) is an established fibrotic biomarker and a well-known mediator of kidney damage. CCN2 was shown to be involved in kidney damage through the regulation of proinflammatory and profibrotic responses. However, to date, the potential role of the NLRP3/RIPK3/NRF2 pathways in CCN2 actions has not been evaluated. In experimental acute kidney injury induced with folic acid in mice, CCN2 deficiency diminished renal inflammatory cell infiltration (monocytes/macrophages and T lymphocytes) as well as the upregulation of proinflammatory genes and the activation of NLRP3/Inflammasome-related components and specific cytokine products, such as IL-1β. Moreover, the NRF2/oxidative pathway was deregulated. Systemic administration of CCN2 to C57BL/6 mice induced kidney immune cell infiltration and activated the NLRP3 pathway. RIPK3 deficiency diminished the CCN2-induced renal upregulation of proinflammatory mediators and prevented NLRP3 modulation. These data suggest that CCN2 plays a fundamental role in sterile inflammation and acute kidney injury by modulating the RIKP3/NLRP3/NRF2 inflammatory pathways.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37627536/
Impact of albumin-lymphocyte-platelet-c-reactive protein index as a prognostic indicator of hepatocellular carcinoma after resection: Associated with nuclear factor erythroid 2-related factor 2
To investigate the prognostic value of preoperative albumin-lymphocyte-platelet-c-reactive protein (ALPC) index in patients with hepatocellular carcinoma (HCC) undergoing curative hepatectomy. We also evaluated with the relationship of the ALPC index and phosphorylated nuclear factor erythroid 2-related factor 2 (p-Nrf2) levels. Data were analyzed retrospectively from 256 patients who underwent resection for HCC. For cross-validation, patients were divided into the training and testing cohort. We assessed eight combinations of inflammatory markers for predictive value for recurrence. We examined the associations of the ALPC index with recurrence-free survival (RFS) and overall survival (OS) in univariate and multivariate analyses (Cox proportional hazards model). Immunohistochemical staining of p-Nrf2 was performed on tumor samples of 317 patients who underwent hepatic resection for HCC. High preoperative ALPC index correlated with a high serum albumin concentration, small tumor size, a low rate of poor differentiation, solitary tumor, early Barcelona Clinic Liver Cancer stage and a low rate of microscopic intrahepatic metastasis in the training dataset. High preoperative ALPC index correlated with a high serum albumin concentration, high serum alpha-fetoprotein concentration, small tumor size, a low rate of poor differentiation and a low rate of microscopic intrahepatic metastasis in the testing dataset. Higher preoperative ALPC index was an independent predictor of longer RFS and OS in the training and testing datasets. High ALPC index was associated with negative p-Nrf2 expression in HCC tumor cells. The authors showed that a high ALPC index was an independent prognostic factor for HCC patients undergoing curative hepatic resection. This article is protected by copyright. All rights reserved.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37632704/
Mammalian SWI/SNF chromatin remodeling complexes promote tyrosine kinase inhibitor resistance in EGFR-mutant lung cancer
Acquired resistance to tyrosine kinase inhibitors (TKI), such as osimertinib used to treat EGFR-mutant lung adenocarcinomas, limits long-term efficacy and is frequently caused by non-genetic mechanisms. Here, we define the chromatin accessibility and gene regulatory signatures of osimertinib sensitive and resistant EGFR-mutant cell and patient-derived models and uncover a role for mammalian SWI/SNF chromatin remodeling complexes in TKI resistance. By profiling mSWI/SNF genome-wide localization, we identify both shared and cancer cell line-specific gene targets underlying the resistant state. Importantly, genetic and pharmacologic disruption of the SMARCA4/SMARCA2 mSWI/SNF ATPases re-sensitizes a subset of resistant models to osimertinib via inhibition of mSWI/SNF-mediated regulation of cellular programs governing cell proliferation, epithelial-to-mesenchymal transition, epithelial cell differentiation, and NRF2 signaling. These data highlight the role of mSWI/SNF complexes in supporting TKI resistance and suggest potential utility of mSWI/SNF inhibitors in TKI-resistant lung cancers.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37541244/
ACTL6A protects gastric cancer cells against ferroptosis through induction of glutathione synthesis
Gastric cancer (GC), one of the most common malignant tumors in the world, exhibits a rapid metastasis rate and causes high mortality. Diagnostic markers and potential therapeutic targets for GCs are urgently needed. Here we show that Actin-like protein 6 A (ACTL6A), encoding an SWI/SNF subunit, is highly expressed in GCs. ACTL6A is found to be critical for regulating the glutathione (GSH) metabolism pathway because it upregulates γ-glutamyl-cysteine ligase catalytic subunit (GCLC) expression, thereby reducing reactive oxygen species (ROS) levels and inhibiting ferroptosis, a regulated form of cell death driven by the accumulation of lipid-based ROS. Mechanistic studies show that ACTL6A upregulates GCLC as a cotranscription factor with Nuclear factor (erythroid-derived 2)-like 2 (NRF2) and that the hydrophobic region of ACTL6A plays an important role. Our data highlight the oncogenic role of ACTL6A in GCs and indicate that inhibition of ACTL6A or GCLC could be a potential treatment strategy for GCs.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37443154/
Therapeutic activity of lipoxin A4 in TiO2-induced arthritis in mice: NF-κB and Nrf2 in synovial fluid leukocytes and neuronal TRPV1 mechanisms
Lipoxin A4 (LXA4) has anti-inflammatory and pro-resolutive roles in inflammation. We evaluated the effects and mechanisms of action of LXA4 in titanium dioxide (TiO2) arthritis, a model of prosthesis-induced joint inflammation and pain. Mice were stimulated with TiO2 (3mg) in the knee joint followed by LXA4 (0.1, 1, or 10ng/animal) or vehicle (ethanol 3.2% in saline) administration. Pain-like behavior, inflammation, and dosages were performed to assess the effects of LXA4 in vivo. LXA4 reduced mechanical and thermal hyperalgesia, histopathological damage, edema, and recruitment of leukocytes without liver, kidney, or stomach toxicity. LXA4 reduced leukocyte migration and modulated cytokine production. These effects were explained by reduced nuclear factor kappa B (NFκB) activation in recruited macrophages. LXA4 improved antioxidant parameters [reduced glutathione (GSH) and 2,2-azino-bis 3-ethylbenzothiazoline-6-sulfonate (ABTS) levels, nuclear factor erythroid 2-related factor 2 (Nrf2) mRNA and Nrf2 protein expression], reducing reactive oxygen species (ROS) fluorescent detection induced by TiO2 in synovial fluid leukocytes. We observed an increase of lipoxin receptor (ALX/FPR2) in transient receptor potential cation channel subfamily V member 1 (TRPV1)+ DRG nociceptive neurons upon TiO2 inflammation. LXA4reduced TiO2-induced TRPV1 mRNA expression and protein detection, as well TRPV1 co-staining with p-NFκB, indicating reduction of neuronal activation. LXA4 down-modulated neuronal activation and response to capsaicin (a TRPV1 agonist) and AITC [a transient receptor potential ankyrin 1 (TRPA1) agonist] of DRG neurons. LXA4 might target recruited leukocytes and primary afferent nociceptive neurons to exert analgesic and anti-inflammatory activities in a model resembling what is observed in patients with prosthesis inflammation.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/37388729/


