Thursday, October 12, 2023
- 08:00 – 09:00 Registration | Poster Mounting
- 09:00 – 13:00 Management Committee Meeting | Hybrid format | MC members only. Chairs: Antonio Cuadrado, Spain, and Gina Manda, Romania
- 11:00 – 11:20 Coffee break
- 13:00 – 14:00 Lunch | Registration
- 14:00 – 16:00 Scientific Session I: NRF2 Regulatory Mechanisms Chairs: Anna-Liisa Levonen-Harju, Finland, and Elena Milanesi, Romania
NRF2/KEAP1 axis governs endothelial cell proteostasis
A. Kopacz1, D. Kloska1, M. Targosz-Korecka2, B. Zapotoczny3, D. Cysewski4, N. Personnic1, E. Werner1, K. Hajduk1, A. Jozkowicz1, A. Grochot-Przeczek1
1 Department of Medical Biotechnology, Faculty of Biochemistry Biophysics and Biotechnology, Jagiellonian University, Krakow, Poland
2 Department of Physics of Nanostructures and Nanotechnology, Institute of Physics, Jagiellonian University, 30-387 Krakow, Poland
3 Institute of Nuclear Physics, Polish Academy of Sciences, 31-342, Krakow, Poland 4 Mass Spectrometry Laboratory, Institute of Biochemistry and Biophysics, Polish Academy of Science, Warsaw, Poland
Email: anna.grochot-przeczek@uj.edu.pl
The gradual decline of cellular protein homeostasis (proteostasis) is a hallmark of ageing and age associated disorders, which is well-established in neurodegeneration. Loss of redox balance is postulated to contribute to this process. NRF2 is a stress-responsive transcription factor repressed by a redox sensitive protein KEAP1. NRF2 levels and its activity may decline with age. We aimed to investigate the proteostasis status in aged and NRF2-deficient endothelial cells and aorta.
We found that aged donor-derived and prematurely senescent NRF-deficient primary human ECs, but not those overexpressing dominant-negative NRF2, exhibit increased accumulation of protein aggregates. A complementary phenotype is observed in the aortas of aged mice and young mice devoid of NRF2 signaling. Loss of proteostasis in the vascular system depends on KEAP1. We show that KEAP1 serves as a critical component of the machinery responsible for proteostasis control. Deposition of protein aggregates can be counteracted by KEAP1 depletion, S-nitrosothiol reductant or rapamycin treatment.
This study shows that vascular system can be affected by protein aggregation and that this process is governed by KEAP1.

Anna Grochot-Przeczek is an associate professor in the Department of Medical Biotechnology, Faculty of Biochemistry, Biophysics, and Biotechnology, Jagiellonian University in Krakow, Poland. She studies the molecular mechanisms that regulate the function of endothelial cells and blood vessels with a focus on NRF2/KEAP1 pathway, ageing and S-nitrosation. Currently, she investigates the importance of NRF2/KEAP1 imbalance and loss of proteostasis in the function of blood vessels.
Elucidating the Protein-Protein Interaction between NRF2 and Pin1: Implications for Cancer Biology and Therapeutic Targeting
Adem Ozleyen1,2, Serhat Donmez3, Mehmet Özbil4, Richard G Doveston1,2, Tugba Boyunegmez Tumer5
1 Leicester Institute for Structural and Chemical Biology, University of Leicester, Leicester, LE1 7RH United Kingdom
2 School of Chemistry, University of Leicester, Leicester, LE1 7RH United Kingdom
3 Graduate Program of Molecular Biology and Genetics, School of Graduate Studies, Canakkale Onsekiz Mart University, Canakkale, 17020 Turkey
4 Institute of Biotechnology, Gebze Technical University, Gebze, Kocaeli, 41400 Turkey 5 Department of Molecular Biology and Genetics, Faculty of Arts and Science, Canakkale Onsekiz Mart University, Canakkale, 17020 Turkey
Email: tumertb@gmail.com, tumertb@comu.edu.tr
Cellular signaling pathways are intricately regulated by protein-protein interactions (PPI), playing a pivotal role in various biological processes, including cancer development. Our study aims to shed light on the complex interplay between NRF2 and Pin1 proteins, as well as the latent binding competition involving Pin1/NRF2/KEAP1. NRF2’s aberrant activation has been implicated in multiple cancer types, driving cell proliferation, suppressing apoptosis, enhancing cancer stem cell self -renewal, and promoting chemoresistance and radioresistance. Likewise, Pin1, a cis-trans isomerase, has been implicated in cancer pathogenesis through its modulation of critical cellular pathways.
Although previous studies hinted at a functional interaction between Pin1 and NRF2, the direct physical nature of these interactions remained elusive. Recently, it was demonstrated that Pin1 indeed interacts with NRF2 and KEAP1 via its WW and PPIase domains, with a specific preference for phosphorylated residues on NRF2. The study revealed the essential role of NRF2 phosphorylation at S215, S408, and S577 for the Pin1-NRF2 interaction. To gain comprehensive insights, our research employs an integrated computational and biophysical approach. We aim to identify potential interaction between 12-mer long peptides, mimicking distinct NRF2 regions (S215, S408, and S577), and Pin1 through molecular docking and dynamics simulations, determining their binding modes. Additionally, qualitative data on the strength and thermodynamic parameters of these interactions by using fluorescence polarization (FP) and isothermal titration calorimetry (ITC) were also identified. Furthermore, we explored the impact of Pin1 inhibitors (juglone, EGCG, KPT-6566, and sulfopin) on this specific PPI, utilizing a combination of biophysical and cell-based methods.
The findings from our study will enhance the understanding of the mechanistic basis of the Pin1-NRF2 interaction, revealing potential avenues for therapeutic targeting of NRF2-driven cancer pathways. We anticipate that our integrated approach will contribute valuable insights to the field of PPIs and offer promising opportunities for the development of novel cancer treatments.

Tugba Boyunegmez Tumer is currently Full Professor of Biochemistry at the Department of Molecular Biology and Genetics, at the Çanakkale Onsekiz Mart University, Turkey. She earned her MSc and PhD degrees from the Middle East Technical University, Turkey in 2004 and 2009, respectively. In 2013 and 2014, she worked as a post-doc researcher in NIH Funded International Research Training Center for Botanicals and Metabolic Syndrome, at Rutgers University, New Jersey, USA. Upon her return to Turkey, she was recruited to Çanakkale Onsekiz Mart University, where she has established a research team focusing on understanding the molecular effects of natural product-derived and synthetic small molecules on chronic inflammatory conditions and is working towards development of pharmacological lead compounds for prevention and treatment of chronic diseases particularly, cancer and neuroinflammation. Her lab combines in vitro/in vivo assays, multi
omic tools and in silico computation, modelling and ADMET approaches for rational lead discoveries.
How can modulation of NRF2 affect Aquaporins?
Monika Mlinarić1, Ivan Lučić1, Ana Josipa Jerončić2, Iva Žulj2, Lidija Milković1, Ana Čipak Gašparović1
1 Division of Molecular Medicine, Ruđer Bošković Institute, Zagreb, Croatia
2 Faculty of Science, University of Zagreb, Zagreb, Croatia
Email: acipak@irb.hr
Aquaporins (AQPs) are membrane pores that facilitate the transport of water and other small polar molecules accross cell membranes. In the course of research into aquaporins,it became apparent ho important it is for their function to channel other molecules in addition to water. Hydrogen peroxide is an important aquaporin substrate that plays a role in cellular signaling and regulates the cell faith decisions. The importance of regulating H2O2 channelling is reflected in the fact that these aquaporins are called peroxiporins. One of the mechanisms by which H2O2 contributes to cell fate regulation is the NRF2 pathway. NRF2 is a master antioxidant transcription factor that is highly activated in breast cancer. Therefore, understanding the regulation of intracellular H2O2 is important for understandiing the responce fo cancer cells to therapy-induced oxidative stress. Here, we show how activation and inhibition of NRF2 affects breast cancer cell lines representing different breast cancers and the impact on aquaporin expression. Additionally, we modulated expression of aquaporins and studied effets on several signaling pathways with emphasis on NRF2. Our results indicate the potential interplay between NRF2 and aquaporins in breast cancer.

Ana Čipak Gašparović is a Senior Research Associate in the Division of Molecular Medicine at the Ruđer Bošković Institute in Zagreb, Croatia. Her research focuses on the role of oxidative stress and antioxidative response in the resistance to cancer treatment. Recently, her research included aquaporins in breast and colon cancer. Special emphasis is given to peroxiporins, specific aquaporins which, in addition to water and glycerol, channel hydrogen peroxide, and as a consequence contribute to oxidative and antioxidative response of the cell. She is interested in the regulation of NRF2 pathway in response to peroxiporins, and their influence on the development of therapy resistance.
Investigating the effects of G-quadruplex structures in NRF2
Hatice Esenkaya
University of Kilis
Email: hatice.esenkaya@kilis.edu.tr
Background: Nuclear factor erythroid 2-related factor 2 (NRF2) is encoded by human NFE2L2 gene and has a role as a transcription factor. This family of proteins regulate expression of antioxidant proteins, Phase II detoxification enzymes and other cytoplasmic enzymes. Studies have shown that the activation of NRF2 is a potential therapeutic target for inflammatory diseases, however inhibition of NRF2 also benefits due to its resistance to some tumour types. The sequence of NRF2 gene around the promoter region sites and the 5’ untranslated region (UTR) of NFR2 mRNA has been shown to contain a number of G tracts and C tracts. Based on bioinformatics analysis some of these regions have the potential to form G-quadruplexes (G4s), four-stranded structures formed by the interaction of such G rich sequences. G4s may affect various DNA and RNA processing reactions, including telomere lengthening to DNA replication, transcription, translation, and splicing. Since G4s are located in guanine rich regions, telomeric regions, gene promoters, and 5’ UTRs, their formation could be a key regulator of aging and age-related degeneration, along with antioxidant processes such as activation of NRF2. Moreover, the existence of G4 structures in NRF2 mRNA may affect NRF2 protein translation. Therefore, exploration and modulating of G4 formation in NRF2 DNA or RNA and their interactions with small molecule ligands has been seen as a possible route to regulating antioxidant system and other pathological systems.
Methods: QUADPARSER, CD (circular dichroism)in the presence of KCl or LiCl.
Results: The preliminary data shows potential existence of G4 in NRF2.
Conclusions: It is shown that G4s are important for the regulation of transcription and translation. The preliminary data shows that NRF2 has potential to form these structures, and could be manipulated to activate or deactivate NRF2 gene by the use of G4 stabilisers. Further investigations are required to confirm whether NRF2 contain G4 stuctures, and to charcterise their role in transcription an translation processes.

Hatice Esenkaya recently graduated with a PhD from the Department of Molecular and Cell Biology at the University of Leicester, UK, supervised by Professor Ian Eperon and Dr Cyril Dominguez, and working on RNA splicing. My recent research was studying the effects of the G-quadruplex stabilising ligand GQC-05 on alternative splicing of Mcl-1 pre-mRNA. My interests are in pre-mRNA splicing and the interaction between spliceosome proteins and RNA. I am particularly interested in how their interaction affect the outcome of alternative splicing. and if their interactions can be altered, using a variety of methods, to see if the splicing pattern can be shifted to obtain more favourable isoform.
Defining the role of the NRF2 transcription factor in synaptic maintenance in Alzheimer’s disease
Daniel Carnicero-Senabre¹, Mariana A Barata², Cláudia Guimas Almeida², Ana I. Rojo¹
1 Department of Biochemistry and Instituto de Investigaciones Biomédicas Alberto Sols UAM-CSIC, Faculty of Medicine, Autonomous University of Madrid, Madrid, Spain. Centro de Investigación Biomédica en Red Sobre Enfermedades Neurodegenerativas (CIBERNED), Instituto de Investigación Sanitaria La Paz (IdiPaz) 2iNOVA4Health, CEDOC, NOVA Medical School, NMS, Universidade Nova de Lisboa, Lisboa, Portugal
Email: airojo@iib.uam.es
Alzheimer’s disease (AD) is characterized by the progressive decrease in the number of synapses within the neuronal circuits. In this study, we will focus on the fact that oxidative alteration of lipids (LPO), crucial components of the synaptic vesicle cycle, neurotransmitter release, and signaling pathways, might deeply impact in synapsis homeostasis. Transcription factor NRF2 regulates the expression of over 250 genes, including those related to protection against oxidative stress. Employing microarray technology, we have analyzed 25,000 transcripts in brain samples from a proprietary AD mouse model based in transgene expression of hAPP(V717I) and TAU(P301L) in a wildtype or null background for NRF2 expression. Comparison of the results from both genotypes identified 6 out of 8 significantly enriched clusters, whose expression was altered by the absence of NRF2 and are related indirectly or directly to synapsis homeostasis. Since we have previously described that absence of NRF2 exacerbates LPO production in connection with a reduction to synaptic transmission in the neurons of the dentate gyrus in these mice, we next evaluated the levels of 544 distinct lipid species from 26 lipid subclasses in NRF2-null or wildtype samples by untargeted lipidomics. We found that NRF2 deficiency leads to the dysregulation of several lipid species from phospholipid, neutral lipids, and from sphingolipids subclasses. Interestingly, the levels of 3 ether-linked phospholipids (plasmalogen, associated with AD human pathology) were increased in the hippocampus of NRF2 null mice compared with the wildtype littermates. To examine synaptic contacts, we analyzed the colocalization of vGLUT1 and PSD95 or vGAT and GEPHYRIN, respective markers of excitatory or inhibitory synapses, both in brain slices and primary neuronal cultures. Our findings revealed that the absence of NRF2 modified the molecular composition of the synapse and caused alterations in the quantity and size of both types of synaptic contacts. Relevantly, pharmacological activation of NRF2 increased the number of vGLUT1/PSD95 positive contacts in primary neuronal cultures. In conclusion, NRF2 emerged as a crucial modulator of synaptic homeostasis, providing a new avenue for exploring its potential as a therapeutic target for neurodegenerative diseases characterized by progressive synaptic loss, such as AD.

Ana I Rojo. Ana I Rojo studied Biochemistry and Molecular Biology at the Autonomous University of Madrid (2001 and 2002), holds a PhD in Biochemistry (graduated in 2006), and since 2017 is professor in Biochemistry at the Autonomous University of Madrid (Faculty of Medicine). As professor, she has participated in multiple teaching activities for the degrees of Biochemistry, Medicine, and Nursing, with special focus on research training. She has been holder of different competitive fellowships and contracts. Her professional career is focused on the study of the molecular basis of neurodegenerative diseases and in the search for novel brain protective therapies with a special focus on redox biology and NRF2 transcription factor. Nowadays, she is exploring the role of NRF2 in the pathogenesis of Alzheimer’s disease and lateral amyotrophic sclerosis as principal investigator. She has published over 50 primary and review articles and participated in more than 30 congresses.
- 15:40 – 16:00 Coffee Break | Poster Viewing
- 16:00 – 18:00 Scientific Session II: NRF2 Monitoring, Drug Targets and Delivery Chairs: Monika Jakubowska, Poland, and Elke Heiss, Austria
The utility of the cellular thermal shift assay for monitoring target engagement by Keap1-Nrf2 protein-protein interaction inhibitors
Annamarie J. Cafferkey, Sharadha Dayalan Naidu, Dina Dikovskaya, Albena T. Dinkova-Kostova
Division of Cellular and Systems Medicine, School of Medicine, University of Dundee, Dundee, United Kingdom
Email: a.dinkovakostova@dundee.ac.uk
Alzheimer’s disease (AD) is characterized by the progressive decrease in the number of synapses within the neuronal circuits. In this study, we will focus on the fact that oxidative alteration of lipids (LPO), crucial components of the synaptic vesicle cycle, neurotransmitter release, and signaling pathways, might deeply impact in synapsis homeostasis. Transcription factor NRF2 regulates the expression of over 250 genes, including those related to protection against oxidative stress. Employing microarray technology, we have analyzed 25,000 transcripts in brain samples from a proprietary AD mouse model based in transgene expression of hAPP(V717I) and TAU(P301L) in a wildtype or null background for NRF2 expression. Comparison of the results from both genotypes identified 6 out of 8 significantly enriched clusters, whose expression was altered by the absence of NRF2 and are related indirectly or directly to synapsis homeostasis. Since we have previously described that absence of NRF2 exacerbates LPO production in connection with a reduction to synaptic transmission in the neurons of the dentate gyrus in these mice, we next evaluated the levels of 544 distinct lipid species from 26 lipid subclasses in NRF2-null or wildtype samples by untargeted lipidomics. We found that NRF2 deficiency leads to the dysregulation of several lipid species from phospholipid, neutral lipids, and from sphingolipids subclasses. Interestingly, the levels of 3 ether-linked phospholipids (plasmalogen, associated with AD human pathology) were increased in the hippocampus of NRF2 null mice compared with the wildtype littermates. To examine synaptic contacts, we analyzed the colocalization of vGLUT1 and PSD95 or vGAT and GEPHYRIN, respective markers of excitatory or inhibitory synapses, both in brain slices and primary neuronal cultures. Our findings revealed that the absence of NRF2 modified the molecular composition of the synapse and caused alterations in the quantity and size of both types of synaptic contacts. Relevantly, pharmacological activation of NRF2 increased the number of vGLUT1/PSD95 positive contacts in primary neuronal cultures. In conclusion, NRF2 emerged as a crucial modulator of synaptic homeostasis, providing a new avenue for exploring its potential as a therapeutic target for neurodegenerative diseases characterized by progressive synaptic loss, such as AD.

Albena Dinkova-Kostova is a Professor of Chemical Biology at the University of Dundee School of Medicine (UK). She graduated in Biochemistry and Microbiology from Sofia University (Bulgaria) and obtained her PhD degree in Biochemistry and Biophysics from Washington State University (USA). She subsequently trained in Pharmacology at Johns Hopkins University School of
Medicine (USA), where she continues to hold an Adjunct Professor position. She joined the University of Dundee in 2007 as a Research Councils UK Academic Fellow. Her group collaborates with basic scientists and clinicians, and with the pharmaceutical industry. In her research, at the interface of Chemical Biology and Medicine, she is committed to understanding how cells and organisms respond to oxidative, inflammatory, and metabolic stress, and is working towards development of strategies for protection against chronic disease. She was named among the top influential academics in Clarivate’s Highly Cited Researchers 2019, 2020, 2021 and 2022 lists.
Development of SIMOA-based kit to determine Nrf2 level
Aysen Cotuk1,2, Ender Avci1, Burak Ibrahim Arioz1,2, Sermin Genc1,3, Sibel Kalyoncu1
1Izmir Biomedicine and Genome Center, Izmir, Turkiye
2Izmir International Biomedicine and Genome Institute, Dokuz Eylul University, Izmir, Turkiye 3 Department of Neuroscience, Institute of Health Sciences, Dokuz Eylul University, Izmir, Turkiye
Email: sibel.kalyoncu@ibg.edu.tr
Nrf2 is a transcription factor playing roles in the intracellular signaling mechanism protecting against oxidative stress. Disruption of Nrf2 pathway appears in the pathogenesis of many inflammatory diseases including diabetes, atherosclerosis, and neurodegenerative diseases. Determination of Nrf2 level and activity has the potential to be used in basic research, as well as in clinical studies for diagnosis, evaluation of drug efficacy and follow-up. In this research, we aim to develop a kit based on Single molecule array (SIMOA) technology that can measure Nrf2 activity and level, which gives much more accurate results than its equivalent ELISA kits. The SIMOA method is a highly sensitive microparticle-based technology, also known as digital ELISA. The method is based on the capture of low amounts of proteins by specific antibodies coated on paramagnetic beads.
To develop a kit that detects Nrf2 level, two anti-Nrf2 antibodies targeting different/distant epitopes should be used for the sandwich approach. Beads coated with those selected antibodies will be used to determine the Nrf2 level. Three-dimensional structure of Nrf2 is not known yet. First, we computationally modelled Nrf2 structure. Then, a panel of commercial anti-Nrf2 antibodies were selected based on their potential Nrf2 epitopes. We screened binding kinetics of four antibodies by Surface Plasmon Resonance (SPR) and found out that most of them binds to Nrf2 quite strongly (dissociation constants in picomolar range). Besides affinity, non-competitive binding through distant Nrf2 epitopes is another important parameter for our SIMOA-based assay. For that purpose, we performed competitive binding assays by using both SPR and ELISA. We successfully selected two anti-Nrf2 antibodies which bind to different epitopes of Nrf2. After this SIMOA based kit is developed and verified, it will be tested with cell-based in vitro assays and Alzheimer’s patient samples in comparison with traditional ELISA assay.

Sibel Kalyoncu Uzunlar graduated from Molecular Biology & Genetics and Chemistry departments in Boğaziçi University (Istanbul, Turkiye) with a double major in 2008. After receiving her master’s degree on Chemistry and Biological Engineering from Koç University (Istanbul, Turkiye) in 2010, she started her PhD at Georgia Institute of Technology (Atlanta, GA, USA). In 2016, she completed her doctorate with her studies in the fields of protein biochemistry and structure. Then, she started her postdoctoral research in the field of antibody engineering at Rensselaer Polytechnic Institute. Her research in the USA has been funded by both public (NIH, NSF) and industry (Novo Nordisk). Returning to Turkey in 2018, she established the Antibody Engineering laboratory as the research group leader at Izmir Biomedicine and Genome Center. By the help of government- and industry supported projects in her laboratory, she aims to develop innovative therapeutic/diagnostic products based on antibody, protein and enzyme engineering.
Enabling Precision: Chemogenetic Tools for Ultralocal Redox- and pH-Control in Nrf2 Pathways
Asal Ghaffari Zaki, Mohammad Miri, Şeyma Çimen, Emrah Eroğlu
Regenerative and Restorative Medicine Research Center (REMER), Research Institute for Health Sciences and Technologies (SABITA), Istanbul Medipol University, Istanbul 34810, Türkiye
Email: emrah.eroglu@medipol.edu.tr
We present a pioneering chemogenetic advancement, focusing on a novel approach for ultralocal pH manipulation. Contrary to conventional understanding, we have revealed the intricate interplay between hydrogen peroxide (H2O2) and Nrf2 activation yet cellular pH in this relationship remains enigmatic. Our chemogenetic tools offer targeted enzyme-based pH modulation within subcellular compartments, enabling controlled pH dynamics. By exploiting these tools, we hope to open a new avenue for investigating Nrf2 signaling complexities. Our modest aim is to shed light on the potential of ultralocal pH manipulation and its impact on Nrf2-related studies.

Emrah Eroğlu serves as a Principal Investigator at Istanbul Medipol University and is the Deputy Director of the Research Institute for Health Sciences and Technologies (SABITA) in Turkey. With a background in biotechnology and molecular biology, his research primarily focuses on investigating intricate cellular processes at the single-molecule level. At Istanbul Medipol University, Dr. Eroğlu’s lab is at the forefront of developing genetically encoded biosensors and chemogenetic tools. These cutting-edge advancements enable the visualization of reactive oxygen and nitrogen species pathways in vascular cells, facilitating a deeper understanding of their roles in signaling cascades and neurodegenerative diseases. Having held significant positions at institutions such as Harvard Medical School and MedUni Graz, Dr. Eroğlu has garnered recognition through honors like the EMBO Installation Grant and the Leopold Flohé Redox Pioneer Young Investigator Award. He actively participates in scientific organizations (SFRR-E) and contributes to esteemed journals such as Redox Biology, FRBM, Redox biology biochemistry and chemistry.
Innovative Drug Delivery Platforms:
Tailoring Carbon Nano-onions for Targeted Therapeutic Applications
Silvia Giordani
School of Chemical Sciences, Dublin City University, Glasnevin, Dublin 9, Ireland
Email: silvia.giordani@dcu.ie
Numerous challenges accompany conventional drug delivery methods, such as adverse side effects, multi-drug resistance, premature drug degradation, inadequate tissue penetration, and non-specific toxicity. Targeted delivery, employing nanocarriers as transport vessels for payloads, holds promise in mitigating these critical concerns. This strategy involves functionalising nanoparticles with targeting agents, which enables their selective uptake by cells that overexpress specific receptors, such that they can transport the therapeutic payload across the cellular membrane of target neoplastic cells. Consequently, this approach enhances drug concentration in the target cells while simultaneously reducing the exposure of healthy cells to the therapeutic agent.
In this oral presentation, carbon nano-onions (CNOs) will be introduced as a promising candidate for nanocarrier-based drug delivery systems. CNOs, also known as multi-layer fullerenes, comprise multiple concentric layers of sp2 hybridised carbon. Our research focuses on the synthesis and functionalisation of CNOs to investigate their potential in drug delivery. A novel CNO-based nanocarrier, incorporating hyaluronic acid— an optimised targeting agent—for the specific delivery of gemcitabine to cells overexpressing CD44 receptors has been developed in my lab (Fig 1). After examining CD44+ and CD44- human pancreatic adenocarcinoma (PDAC) cells, we observed that fluorescently labelled CNOs were selectively internalised by CD44+ cells, with no significant toxicity detected within a biologically significant concentration range. We demonstrate that CNOs possess low toxicity and could potentially be employed to deliver gemcitabine directly to CD44+-PDAC cells. Our results highlight this; the CNO-based nanocarriers demonstrated excellent in vitro outcomes in PDAC cells that are otherwise particularly difficult to treat due to various chemoresistance-pertinent factors.
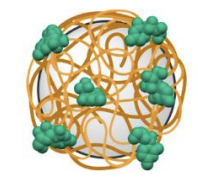
Fig 1. Schematic of a CNO-based (grey) nanocarrier designed to deliver gemcitabine (green) to tumours via hyaluronic acid (orange) mediated targeting of cancer cells overexpressing CD44 receptors.
Silvia Giordani joined the School of Chemical Sciences at Dublin City University as Professor Chair of Nanomaterials in 2018. Previously she received her PhD in Chemistry from the University of Miami, USA and carried out postdoctoral research at Trinity College Dublin (TCD) and at the University of Trieste, Italy. In 2007 she received the prestigious President of Ireland Young Researcher Award and was a Research Assistant Professor at TCD from 2007 to 2013. In 2013 she founded and directed the new “Nano Carbon Materials” research lab at the Istituto Italiano di Tecnologia (IIT) and in December 2016 she was appointed Associate Professor in Organic Chemistry at the University of Turin, Italy. Her main research interests are in the design, synthesis, and characterization of a wide range of nanomaterials for applications in smart and responsive bio related nanotechnologies. She is the author/co-author of more than 150 manuscripts, reviews and book chapters. She is the recipient of many international prizes and honours including the L’Oreal UNESCO for Women in Science fellowship, the William Evans visiting fellowship from the University of Otago (New Zealand) and is a Visiting Scientist to the Bio-Nano Institute at Toyo University (Japan). Her research has been recently featured in “Where I work” published in Nature on the 20th May 2021.
Adera2.0: A Drug Repurposing Platform Centered on NRF2
Norwin Kubick1, Jarosław Olav Horbańczuk2, Mariusz Sacharczuk2,3, Michel Mickael3
1 Department of Biology, Institute of Plant Science and Microbiology, University of Hamburg, Ohnhorststr. 18, 22609, Hamburg, Germany
2 Department of Pharmacodynamics, Faculty of Pharmacy with the Laboratory Medicine Division, Medical University of Warsaw, Banacha 1B, 02-091 Warsaw, Poland
3 Institute of Genetics and Animal Biotechnology of the Polish Academy of Sciences, ul. Postepu 36A, Jastrzebiec, 05-552 Magdalenka, Poland
Email: michel.edwar77@gmail.com
In the realm of neuroimmunological (NI) investigations, the early stages of drug repurposing are unfolding with a specific focus on targeting the NRF2 pathway. This approach circumvents the protracted processes of drug discovery, focusing instead on uncovering novel applications for established medications that can modulate the NRF2 pathway. Neuroimmunological diseases—such as Alzheimer’s, Parkinson’s, multiple sclerosis, and depression—emerge from intricate interactions between the central nervous system and the immune system. A large amount of research has shown that NRF2 plays a crucial role in NI diseases. Yet, repurposing existing drugs to influence the NRF2 pathway faces challenges due to the extensive information requiring extraction. In a previous iteration, Adera1.0 showcased its prowess in text mining PubMed to answer query-based inquiries. Through this approach we showed that zilution can be used to activate NRF2. However, Adera1.0 lacked the capacity to autonomously pinpoint chemical compounds. To address the imperative of repurposing established medications for neuroimmunological conditions with NRF2 modulation, we devised Adera2.0—a deep neural network primed for drug repurposing with a specific emphasis on NRF2 modulators. This innovative workflow encompasses three deep learning networks. The initial network operates as an encoder, embedding text into matrices. The subsequent network employs a mean squared error (MSE) loss function, forecasting responses in the form of embedded matrices. The third network, the keystone of our refined workflow, also leverages an MSE loss function. Its primary role involves extracting compound names from sentences derived from the previous network that are associated with could have the capacity to modulate NRF2. Through meticulous optimization, we assessed eight distinct designs, culminating in a profound neural network comprising an RNN neural network and a leaky ReLU activation function, yielding a 0.0001 loss and 67% sensitivity. Furthermore, we subjected Adera2.0 to validation against the DRUG Repurposing Hub database, affirming its aptitude to predict drugs that could be used to target the NRF2 pathway. These outcomes affirm Adera2.0’s capability to potentially expedite drug development cycles with a focus on NRF2- related therapeutics. The workflow is readily accessible online, providing a valuable resource for drug repurposing endeavors aimed at NRF2 modulation.
Clinical Trials in cardiometabolic research
Harald Sourij1,2, Heiko Bugger3, Andreas Zirlik3, Hans-Peter Dimai2
1Interdisciplinary Metabolic Medicine Trials Univ, Medical University of Graz
2 Div. of Endocrinology and Diabetology, Medical University of Graz
3 Division of Cardiology, Medical University of Graz, Austria
Email: ha.sourij@medunigraz.at
Obesity, diabetes, cardiovascular disease (CVD) and heart failure are intertwined diseases, altogether representing leading causes of health care expenditures and mortality in industrialised countries. In Austria, direct and indirect annual costs for CVD were estimated to be 5 billion Euros, highlighting the importance of developing multi-omic research initiatives to better understand the interrelation of cardiometabolic diseases, improve risk stratification, elucidate underlying pathophysiological and treatment mechanisms, and design interventions to reduce disease burden.
Blood based biomarkers could play an important role in guiding risk factor management. Beyond traditional risk factors, a residual cardiovascular CV risk remains, defined as the remaining risk of CV events or progression of CV disease that persists despite treatment according to evidence-based guideline recommendations. Analyses of various cohorts showed that high-sensitivity CRP (hsCRP) indicates a residual inflammatory risk that predicts CV events independently of LDL cholesterol, and that the contribution from LDL and hsCRP on CV risk are additive.
Clinical trials represent the gold standard to investigate the impact of pharmacological and non pharmacological interventions in people with cardiometabolic conditions. A currently planned and EU supported multicenter trial will investigate, if an intensified risk factor intervention, focusing on inflammation, was beneficial in people after acute coronary syndrome and extremely high CV risk based in biomarker stratification.

Harald Sourij is Full Professor for Interdisciplinary Metabolic Medicine, the Head of the Interdisciplinary Metabolic Medicine Trials Unit, the Head of the Diabetes, Lipid and Metabolism Outpatient Clinics and Deputy Head of the Division of Endocrinology and Metabolism at the Medical University of Graz, Austria. After completing his studies sub auspiciis presidentis (summa cum laude) in 2004 and his training at the Medical University of Graz, Dr Sourij specialised as a consultant in internal medicine, as well as diabetes and endocrinology.
In 2010 Dr Sourij joined the Diabetes Trials Unit lead by Prof Rury Holman at the University of Oxford, UK, where he acted as the clinical lead for the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) until he returned to Graz, Austria in 2013. Since 2020 he is heading the Interdisciplinary Metabolic Medicine Trials Unit specialized in designing and executing mono- and multicenter clinical trials in the cardiometabolic field. Harald has published >185 manuscripts in peer reviewed journals including Lancet, European Heart Journal or Diabetes Care.
- 18:00 – 19:30 Guided City Tour of Old Town of Graz (start: Meeting venue, end: City hall)
- 19:30 – 21:30 Reception by the Mayor of the City of Graz (City Hall)
Friday, October 13, 2023
- 09:00 – 11:00 Scientific Session III: Modulation of NRF2 Chairs: Kattri-Liis Eskla, Estonia, and Ivana Stojanović, Serbia
Oxygen: a key modulator of the Nrf2-antioxidant system
Roberto Motterlini and Roberta Foresti
Faculty of Health, University Paris-Est Créteil, INSERM, Créteil, France
Email: roberto.motterlini@insrrm.fr
Oxygen (O2) is a controversial gaseous molecule. If on one hand O2 is a vital element for mammalian organisms, on the other it is the predominant source for the formation of reactive oxygen species (ROS), thus constituting the most elusive and dangerous poison for living cells. The potential toxicity of this gas is reflected in the fact that the physiological concentrations of free O2 in tissues are maintained at much lower levels (1-2%) compared to the surrounding atmospheric O2 tension, which is around 20%. Despite these undisputable differences, common practice for in vitro work in research laboratories relies on culturing cells in dedicated incubators at atmospheric O2 levels. In this lecture we will examine this fundamental concept to reveal that studying the phenotype of cells grown at concentrations of O2 similar to the ones found in tissues provide important information on the artefacts that we encounter when culturing cells at ambient O2. We first show that, although murine macrophages cultured at 20 and 5% O2 display similar O2 consumption rate and ATP levels, atmospheric O2 (20%) drives the endogenous cellular environment towards an oxidative stress and pro inflammatory phenotype. In particular, compared to cells cultured at 5% O2, cells grown at atmospheric O2 are characterized by higher basal ROS production, decreased levels of reduced glutathione and increased expression of Nrf2-dependent pathways including heme oxygenase-1 and NAD(P)H quinone oxidoreductase. Moreover, when challenged with lipopolysaccharide, cells at 5% O2 display lower levels of pro-inflammatory markers and an increased expression of anti-inflammatory genes. Thus, permanent culture of cells at “physiologically relevant O2 tensions” is warranted in order to properly characterize their redox, metabolic and inflammatory state and, most importantly, their true response to drugs and chemicals.

Roberto Motterlini is Director of Research (DR1) at INSERM U955 within the Faculty of Health, University of Paris Est, France. He has a long-standing interest in the regulation, activity and biological significance of heme oxygenase-1 (HO-1), a ubiquitous defensive protein that degrades heme to carbon monoxide (CO) and biliverdin. His studies focused on the role of Nrf2 as a transcription factor in controlling HO-1 gene expression and uncovered the vasodilatory, anti-ischemic and anti-inflammatory properties of CO. His research led to the development of CO
releasing molecules (CO-RMs), small active compounds that deliver controlled amounts of CO in vivo and have been shown to exert important pharmacological actions to counteract vascular, inflammatory and metabolic disorders. Dr. Motterlini’s group has also characterized a new class of hybrid compounds, termed HYCOs, which have the ability to activate Nrf2 and simultaneously release CO. He is currently investigating whether CO and molecular O2 can compete for the same cellular targets to act as antagonists in the modulation of metabolic dysfunction and inflammatory conditions.
Carbon monoxide: a promising therapeutic against obesity
Roberta Foresti and Roberto Motterlini
University Paris Est Créteil, INSERM, IMRB, F-94010, Créteil, France
Email: roberta.foresti@inserm.fr
Obesity leads to accumulation of adipose tissue and is associated with development of insulin resistance. Carbon monoxide-releasing molecules (CORMs) have been shown to improve metabolic profile in obese animals but the underlying mechanisms are poorly understood. In this work we show that oral administration of CORM-401 to mice fed a high fat diet (HFD) significantly reduced body weight gain and markedly improved glucose homeostasis. We further demonstrates that CO accumulates in adipose tissue and uncouples mitochondrial respiration in adipocytes, resulting in a switch toward glycolysis. This effect was accompanied by enhanced sensitivity to insulin, with lowering of glycemia and increased AKT phosphorylation. We will also present unpublished observations supporting a role of CO in reprogramming the gut microbiota during obesity. In fact, a detailed study to determine CO body distribution after CORM-401 treatment revealed that CO is abundantly localized in the feces, suggesting that CO could also affect the microbiota to combat obesity. Indeed, we observed that mice under HFD develop microbiota dysbiosis compared to mice fed a standard diet and that treatment of with CORM-401 promoted an enrichment of specific bacterial species that are associated with a healthy metabolic profile and are beneficial for the host. Our combined data indicate that, once released from CORM-401, CO reaches several body compartments where it exerts specific positive pharmacological effects against obesity, suggesting that the interaction of CO with multiple sensitive targets in the body could be exploited for therapeutic application in metabolic diseases.

Roberta Foresti (PhD) is Professor of Biochemistry at the Faculty of Health at the University Paris Est Créteil and working as a scientist at the Mondor Institute of Biomedical Research (IMRB). Dr Foresti teaches different subjects, such as human nutrition, energetic metabolism and oxidative stress, to bachelor and master students. She has dedicated more than two decades to investigating the protective action of the heme oxygenase-1 (HO-1) pathway in the cardiovascular system and inflammation, additionally focusing on drug discovery approaches targeting Nrf2/HO-1. She contributed to demonstrate an important role for the HO-1-derived products bilirubin and carbon monoxide (CO) in the protection of cardiovascular function during stress and is currently interested in the study of CO in obesity and metabolism. Dr Foresti is responsible of International Relations at IMRB, organizing a series of activities for the international recognition of our institute, including a summer school on environmental aggressions and health/chronic disease.
Immunomodulatory properties of HYCOs, Nrf2 activators that simultaneously release carbon monoxide (CO) to cells and tissues
Goran Stegnjaić1, Dragica Mićanović1, Neda Nikolovski1, Miljana Momčilović1, Tamara Saksida1, Roberta Foresti2, Roberto Motterlini2, Đorđe Miljković1
1 Department of Immunology, Institute for Biological Research ”Siniša Stanković’’, National Institute of the Republic of Serbia, University of Belgrade, Belgrade, Serbia
2 University Paris Est Créteil, INSERM, IMRB, F-94010, Créteil, France
Email: djordjem@ibiss.bg.ac.rs
HYCOs are a novel class of hybrid compounds consisting of fumaric esters conjugated to carbon monoxide releasing molecules (CO-RMs). They were designed based on the consideration that fumaric esters are known to activate the transcription factor Nrf2 and that CO possesses potent anti-inflammatory properties. The dual action of these hybrids has shown promising therapeutic effects. in animal models of psoriasis and multiple sclerosis. We have recently started with the group of Drs Motterlini and Foresti in France a collaborative research project relevant to the BenBedPharm COST Action, focusing on the immunomodulatory effects of HYCOs. These effects were examined in vitro in cultures of myeloid-derived cells (macrophages and dendritic cells), lymph node cells, immune cells isolated from the inflamed central nervous system, and microglia. By assessing the production of immunoactive molecules, including nitric oxide, reactive oxygen species and cytokines, we provide evidence that HYCOs display immunomodulatory effects in all cell populations examined in vitro. Moreover, we were able to demonstrate that HYCOs are efficient in ameliorating type 1 diabetes in an animal model of this autoimmune disease. Our results indicate that HYCOs are Nrf2 activators with promising immunomodulatory therapeutic properties.
Đorđe Miljković is a Research Professor and the Head of the Department of Immunology at the Institute for Biological Research “Siniša Stanković”, University of Belgrade. He studies cellular and molecular mechanisms involved in the pathogenesis of autoimmune diseases. His current main research interests are: role of gut immune cells in autoimmunity, mechanisms of autoimmunity progression/regulation, cell-based therapy of autoimmunity, modulation of autoimmune diseases by synthetic and natural compounds.
The effect of DPP3 mutation found in cancer on the KEAP1-NRF2 Signaling Pathway
Mihaela Matovina, Sara Matić, Ana Tomašić Paić, Sandra Sobočanec, Marija Pinterić, Sanja Tomić
Division of Organic Chemistry and Biochemistry, Ruđer Bošković Institute, Zagreb, Croatia
Email: mmatovina@irb.hr
Dipeptidyl peptidase 3 (DPP3) is a zinc-dependent peptidase that cleaves off dipeptides from the amino termini of 4-8 amino acids long peptides, with relatively low specificity, in vitro. The physiological roles of DPP3 are not completely elucidated, however, it is presumed that it has a role in the final stages of protein turnover in cells, and in the regulation of blood pressure and pain. It is also involved in the regulation of KEAP1-NRF2 signaling pathway through its interaction with KEAP1. Increased expression or activity of DPP3 have been found in several different cancers, including endometrium, ovary, lung, breast and colorectal cancer. In multiple myeloma and estrogen receptor-positive (ER+) breast cancer, increased DPP3 expression correlates with poor prognosis, and in ER+ breast cancer increased expression of DPP3 also correlates with increased expression of NRF2-controlled genes. The exact mechanism of involvement of DPP3 overexpression in the cancer progression is not clear, but one of the mechanisms may be activation of the NRF2-KEAP1 pathway. We have analyzed several mutants of DPP3 whose genomic sequences were found in cBioPortal for cancer genomic database in order to determine whether the mutations might be involved in cancer progression, either through increased activity or stronger interaction with KEAP1. Mutants selected based on the results of MD simulations of DPP3 binding to the Kelch domain were expressed in E. coli. The purified proteins were tested for their enzyme activity and for their binding to the Kelch domain of KEAP1. Enzyme kinetics analysis revealed no mutants with higher activity than WT DPP3; however, microscale thermophoresis (MST) analysis showed that mutant R623W has more than 100 fold lower Kd for binding to the Kelch domain than WT DPP3. WT and R623W mutant were overexpressed in HEK293T cells, and the effect of their overexpression on the expression of several NRF2-controlled genes was analyzed at both the mRNA and protein levels. Overexpression of the DPP3-R623W resulted in upregulation of NQO1 at both mRNA and protein levels, while upregulation of HMOX1 and NRF2 was detected only at protein level. WT DPP3 also upregulated NQO1 and HMOX1 protein levels, but to a lesser extent. The results of the study suggest that one of the mechanisms of DPP3 involvement in cancer progression could be through the expression of mutated proteins with higher affinity for KEAP1 that cause upregulation of the KEAP1-NRF2 signaling pathway.
Mihaela Matovina is the Senior Research Associate in the Laboratory for Protein Biochemistry and Molecular Modeling at the Division of Organic Chemistry and Biochemistry of Ruđer Bošković Institute in Zagreb, Croatia, and Assistant Professor at Josip Juraj Strossmayer University of Osijek, Croatia. During her PhD, she has studied the molecular epidemiology of human papillomavirus and its involvement in cervical cancer progression and earned her PhD in the field of biology from the Science Faculty of the University of Zagreb in 2006. From 2006 to 2009 she worked as a Postdoctoral Associate at Brown University, Providence RI, USA where she worked in the laboratory of Prof. Arthur Landy on the mechanisms of site specific integration of lambda phage DNA in the bacterial chromosome. Currently, she is investigating pathophysiological role(s) of dipeptidyl peptidase 3 (DPP3). Her main focus are protein-protein interactions of DPP3, including its interaction with KEAP1 which is involved in the regulation of KEAP1-NRF2 pathway. She is also searching for novel interactors of DPP3 in order to gain more insight into its physiological role.
Neuroprotective role of Nrf2 activation in Parkinson´s disease
Noemí Esteras1,2 & Andrey Y Abramov1
1 Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, University College London, UK
2 Department of Biochemistry and Molecular Biology, School of Medicine, Complutense University of Madrid, Spain
Email: nesteras@ucm.es
Parkinson´s disease (PD) is one of the most prevalent neurodegenerative conditions, affecting more than 10 million people worldwide. About 10% of all patients present a genetical form of this movement disorder linked to autosomal dominant mutations in genes such as SNCA, PINK1, Parkin, LRRK2 or DJ-1. PD is characterized by the aberrant aggregation of alpha synuclein (α-SYN) protein and the loss of nigro-striatal dopaminergic neurons, responsible for fine movement control. Oxidative stress and mitochondrial dysfunction are well-known molecular hallmarks of PD and are believed to underlie the neurodegeneration process. Importantly, both have been previously shown to be targeted by pharmacological Nrf2 activation in other disorders.
Here, we have explored the ability of the potent Nrf2 activator omaveloxolone (omav) in recovering the mitochondrial function and antioxidant capacity in human in vitro models of Parkinson´s disease such as fibroblasts obtained from seven patients with the familiar form of the disease harbouring mutations in different genes (SNCA, PINK1, PARK2, DJ-1 and LRRK2). Our results show that omav is able to enhance the antioxidant defence of these cells by increasing glutathione levels both in basal conditio ns and when they are challenged with tert-butyl hydroperoxide, therefore protecting against oxidative stress. In addition, omav improved mitochondrial bioenergetic function as shown by the recovery of the mitochondrial membrane potential, which is typically reduced in the patient´s cells. We show that the underlying reason for this improvement is the role of omav in increasing mitochondrial NADH availability.
Our results show that Nrf2 activation is able to tackle two essential pathogenic pathways in PD: it enhances the antioxidant defence and promotes mitochondrial bioenergetics, opening new avenues for the potential neuroprotective role of omav in PD.
This research is sponsored by Reata Pharmaceuticals, Inc.
Noemí Esteras is currently a recent PI holding a Ramón y Cajal Fellowship at Complutense University of Madrid, Spain, where she first graduated in Pharmacy (2007) and obtained a PhD in Biochemistry and Molecular Biology (2012). She then developed most of her research career as a postdoc (from 2014) and Senior Research Fellow (from 2019) at the Queen Square Institute of Neurology, University College London, UK. Her work is focused in understanding the molecular mechanisms leading to neurodegeneration, and particularly, the interaction of mitochondria, oxidative stress and calcium signalling in the pathogenesis of disease. She is very interested in the role of Nrf2 as a modulator of mitochondrial function, both in brain physiology and as a therapeutic strategy in neurodegeneration, and has published several original and review articles on the topic.
- 10:40 – 11:00 Coffee Break | Poster Viewing
- 11:00 – 11:50 Keynote Lecture Chairs: Antonio Cuadrado, Spain, and Albena Dinkova-Kostova, UK
Molecular Basis of Oxidative Stress Sensing and Its Perturbation
Masayuki Yamamoto
Tohoku University Tohoku Medical Megabank Organization
Email: masayuki.yamamoto.c7@tohoku.ac.jp
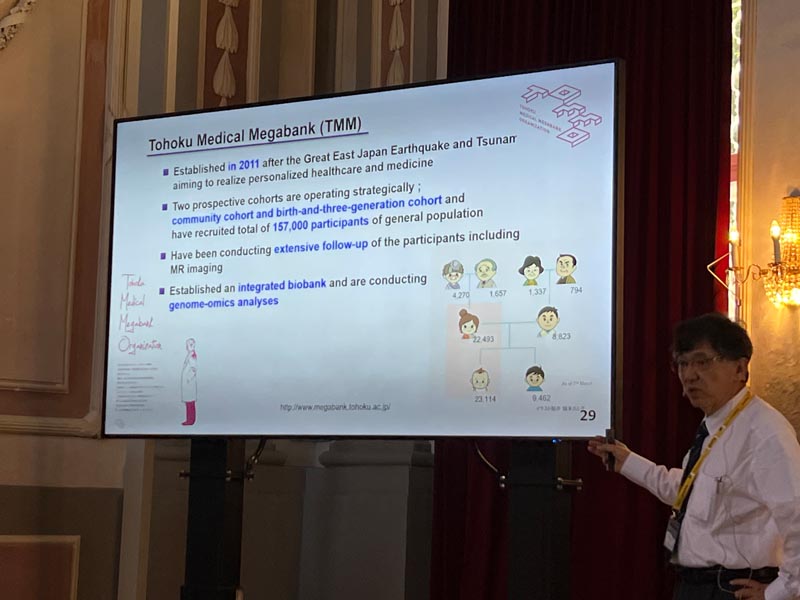
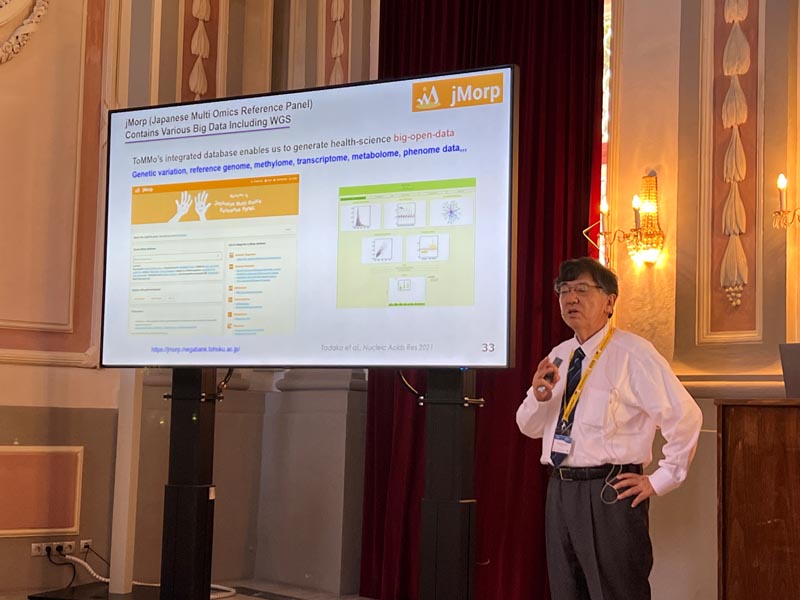
Our bodies possess an ability to detect environmental stresses and activate cellular defenses. In our exploration of lineage determining transcription factors, we discovered the CNC-sMAF transcription factor family and the KEAP1-NRF2 stress response system. Transcription factor NRF2 is crucial for the coordinated expression of enzymes defending against oxidative and electrophilic stresses. KEAP1 acts as a sensor for the stresses and as a subunit of ubiquitin-E3 ligase that degrades NRF2 constitutively. Nrf2 gene knockout animals are vulnerable to a wide variety of toxic electrophiles and reactive oxygen species. In contrast, those with the Keap1 gene knockdown shows a gain-of-function phenotype of the cytoprotection. Modifications in KEAP1 cysteine residues neutralize its ubiquitin ligase activity and stabilize NRF2. This mechanism is referred to as the Cysteine Code. Genetic as well as pharmacological induction of NRF2 protect tissues from oxidative injury. We have demonstrated this protective activity in several disease models, such as experimental autoimmune encephalomyelopathy, sickle cell disease, and acute kidney injury. The field continues to evolve, now encompassing Nrf2 regulation of inflammation, metabolism, ageing and neuroprotection. Research on the KEAP1-NRF2 system is now broadening to include human biology, as seen in cohort studies of Tohoku Medical Megabank, and space mouse biology utilizing International Space Station. This presentation will offer both a historical overview and insights into recent advancements in the KEAP1-NRF2 system.

Masayuki Yamamoto graduated from Tohoku University School of Medicine in 1979 and Graduate School of Medicine in 1983. From 1983 to 1986, he worked as a postdoctoral fellow at Northwestern University under the guidance of Professor Engel. Collaboratively, they identified the GATA family of transcription factors, now widely recognized as a foundational transcription factor family that regulates lineage commitment and cellular differentiation. In 1995, Yamamoto initiated an in depth analysis of the CNC-sMAF family of transcription factors. He had identified and established the KEAP1-NRF2 system, which controls the cellular response to electrophilic and oxidative stresses. His research in this domain continues to break ground. Throughout his career, Yamamoto has been honored with awards, such as: Leading Edge in Basic Science Award (SOT, 2011), Medal of Honor with Purple Ribbon (The Emperor of Japan, 2012), Japan Academy Prize (2014), Award for Research Excellence (FAOBMB, 2020), Lester Packer Award (2021). In a move to assist in the reconstruction efforts following the devastation of the Great East Japan Earthquake, Yamamoto founded the Tohoku Medical Megabank organization in 2012 and currently serving as an Executive Director.
- 11:50 – 13:10 Scientific Session IV: Modulation of NRF2 Chair: Santiago Cuevas Gonzalez, Spain, and Marlene Santos, Portugal
Inhibition of Glutathione S transferase enzymes by NRF2 Activators: An off-target effect that may limit their therapeutic potential
Ankit Kumar1, Jayarman Muthukumaran1 and Chakradhara Rao Satyanarayana Uppugunduri2
1 Department of Biotechnology, Sharda School of Engineering and Technology, Sharda University, Greater Noida, India
2 Onco-Haematology Unit, Department of Paediatrics, Obstetrics and Gynaecology, Geneva University Hospitals, Geneva, Switzerland; CANSEARCH Research Platform on Pediatric Onco-Hematology, Department of Paediatrics, Obstetrics and Gynaecology, Faculty of Medicine, University of Geneva, Geneva, Switzerland
Email: chakradhara.uppugudnuri@unige.ch
The NRF2 signaling pathway is known to regulate various antioxidant response elements, including glutathione s transferase enzymes (GSTs). GSTs play a vital role in detoxification of xenobiotics, endogenous compounds and also in cell cycle proliferation and survival. NRF2 activators, known for their ability to induce antioxidant response elements, including GSTs. NRF2 activators may have an off-target transient direct effect on GST proteins upon acute exposures, different from that of the persistent regulation of the GST genes upon chronic exposures. Understanding this effect by NRF2-activators is crucial for the development of targeted interventions. We implemented, (a)Text Mining: to identify NRF2 activators and extract relevant information from research articles and databases, (b)Molecular Docking: to analyze the interaction between NRF2 activators and GST enzymes, (c)MD Simulation: on the prioritized NRF2 activators from molecular docking studies to assess their stability and dynamics of the interaction,(d)MMPBSA Analysis: to calculate the binding free energy of the prioritized lead candidates, (d) In vitro Testing: Validate the prioritized lead candidates by performing GST enzyme inhibition experiments. Text mining yielded a total of 44 molecules that have been experimentally validated as NRF2 activators and 4 molecules as NRF2-KEAP1 PPI inhibitors. Subsequently, out of them, 41 molecules are known to inhibit GST isoforms either in vitro or in vivo studies. Following we launched molecular docking using 3 positive controls and 3 negative controls and 48 NRF2 activators/NRF2-KEAP1 inhibitors. Overall, the findings from this study contribute to the growing understanding of the relationship between NRF2 activators and their transient GST inhibition in determining therapeutic potential in the context of cellular protection.

Chakradhara Rao S Uppugunduri holds a PhD in Medical Pharmacology. He is currently working at CANSEARCH Research platform of pediatric oncology and hematology of University of Geneva and heading the experimental research at this platform. He is a recognized Clinical Pharmacologist from Swiss Society of Clinical Pharmacology and Toxicology (SSCPT). His research is focused on pharmacogenetics and personalized medicine with particular contributions in elucidating drug responses, drug-drug interactions in relation to genetic variants.
Administration of a sulforaphane-producing supplement to mice via the drinking water over 3 months: analysis of safety and efficacy in activating Nrf2 in various target tissues
Panos G. Ziros1, Georgios Psarias1, Sheng Huang1, Dionysios V. Chartoumpekis1, Massimo Bongiovanni2 and Gerasimos P. Sykiotis1
1 Service of Endocrinology, Diabetology and Metabolism, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
2 Synlab Pathology, Lausanne, Switzerland
Email: gerasimos.sykiotis@chuv.ch
Sulforaphane is one of the best studied Nrf2-activating compounds. In preclinical studies, it is usually administered either intravenously or via gavage. While these administration methods are efficacious in activating Nrf2 in target tissues, they have certain disadvantages: (i) they are invasive and thus not optimal from the perspective of the 3Rs (Replacement-Reduction-Refinement), notably regarding Refinement; (ii) their invasive nature may potentially affect certain molecular or behavioral readouts; (iii) they are labor-intensive for the personnel working with the experimental animals and require proper training to avoid injury to the animals; (iv) they do not recapitulate the preferred mode of administration of drugs to humans (i.e., oral intake). An oral sulforaphane-producing supplement (Avmacol®) in the form of a pill is on the market for human use. Each pill supplies broccoli seed extract that contains the sulforaphane precursor glucoraphanin as well as active myrosinase enzyme that converts glucoraphanin into sulforaphane in the small intestine. Studies in humans have supported that this supplement can effectively activate Nrf2 in vivo. The main objective of the present study was to test whether oral administration of the supplement to mice via the drinking water is safe and efficacious in activating Nrf2 in various target tissues. First, the supplement was dissolved in water and the capacity of the resulting solution to activate Nrf2 was tested by treating cells stably transfected with an ARE luciferase construct. Dose-dependent Nrf2 activation was consistently observed. Maintaining the solution at room temperature for up to 7 days before treating the cells had no effect on its capacity to activate Nrf2 in vitro. Next, male and female adult mice were treated with the supplement, which was dissolved in their drinking water. The mice had continuous access to the supplement solution, which was their only source of drinking water. The solution was changed every 2-3 days, and the mice were treated for a total of 3 months. No adverse events or altered behavior were observed during this period. The mice were then sacrificed, and various tissues were harvested for molecular and histological analyses. A mild induction of Nrf2 activity was observed in the liver and other tissues, as reflected in an increase of Nqo1 mRNA expression levels. At the histological level, no signs of tissue damage were observed. These data demonstrate that administration of a commercially available sulforaphane-producing supplement to mice via the drinking water over 3 months is safe and efficacious in activating Nrf2. This method is then suitable for preclinical studies in the field of Nrf2-related research.

Gerasimos (Gerry) Sykiotis is Senior Staff Physician at Lausanne University Hospital and Associate Professor at the University of Lausanne. He specializes in clinical and basic endocrinology with a particular focus on thyroid physiology and thyroid diseases, including thyroid cancer. Since 2015, he is responsible for the thyroid clinic at the Endocrine Division of Lausanne University Hospital. His basic research, funded primarily by the Swiss National Science Foundation, focuses on the roles of cellular antioxidant response systems in thyroid physiology and pathophysiology. His clinical research focuses on the needs of patients with benign and malignant thyroid diseases.
Dimethyl fumarate does not alter vascular functions in borderline hypertensive rats
Iveta Bernatova1, Michal Kluknavsky1, Andrea Micurova1, Silvia Liskova1,2 Peter Balis1
1 Department of Experimental Hypertension, Institute of Normal and Pathological Physiology, Centre of Experimental Medicine, Slovak Academy of Sciences, Bratislava, Slovakia
2 Faculty of Medicine, Institute of Pharmacology and Clinical Pharmacology, Comenius University, Sasinkova 4, 811 08 Bratislava, Slovakia
Email: Iveta.Bernatova@savba.sk
In humans, a borderline increase in blood pressure (prehypertension) is a common phenomenon already in young age. Prehypertension is a risk factor for other CVDs, especially in the presence of metabolic diseases such as liver diseases, disorders of lipid metabolism and type 2 diabetes. Dimethyl fumarate (DMF) is an activator of NRF2, its possible therapeutic use in CVDs and liver diseases has already been identified. Since DMF has been approved for the treatment of psoriasis and multiple sclerosis in humans, expanding the use of DMF may represent a relatively safe way to activate NRF2-regulated pathways in other diseases, including the CVDs. We investigated changes selected parameters in the heart and liver as well as vascular function in the femoral and mesenteric arteries of adult male borderline hypertensive rats (BHRs) after six-week oral DMF treatment (30 mg/kg/day). DMF did not change systolic blood pressure and heart rate of BHR-treated rats. Plasma alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase were not affected by DMF. However, plasma lactate dehydrogenase and α-hydroxybutyrate dehydrogenase were significantly elevated in DMF-treated rats, which was associated with elevated relative left heart ventricle (LHV) weight. Nitric oxide (NO) synthase activity was significantly elevated in the LHV and liver but no changes were found in the aorta. Endothelium-dependent acetylcholine (ACh)-induced and sodium nitroprusside-induced relaxations in the femoral arteries and mesenteric arteries were similar in the control and DMF-treated rats and no changes in NO-dependent and NO-independent components of ACh-induced relaxation were found between the groups after acute L-NAME-pretreatment of the arteries. Similarly, no significant differences in the contractile functions of these arteries were found between the groups. The expression of Nfe2l2, Hmox-1, Sod1 and Gpx4 genes were elevated in the LHV. On the other hand, Nfe2l2 was significantly reduced in the liver, without changes in Hmox-1, Sod1 and Gpx4 expressions. In the aorta, Nfe2l2 and Nos3 were not affected by DMF. In conclusion, our results showed that DMF does not affect blood pressure in rats with prehypertension and it has tissue-dependent effects on the expression of Nfe2l2 in the LHV, aorta and liver. Furthermore, DMF did not affect femoral and mesenteric arteries functions in the model of borderline hypertension which was associated with unchanged Nfe2l2 and Nos3 gene expression. This study was supported by grants Nos. VEGA-2/0157/21, APVV-22-0296 and MVTS-COST-CA20121.

Iveta Bernatova is a senior scientist, head of the Department of Experimental Hypertension at the Institute of Normal and Pathological Physiology, Centre of Experimental Medicine, Slovak Academy of Sciences, Bratislava, Slovakia. She studied Biochemistry (1991), holds Ph.D. from Chemistry (1997) and a title Doctor of Sciences (D.Sc.) in the field of Animal Physiology (2009). Her research is focused on the regulatory mechanisms of blood pressure in various experimental models of hypertension and the ways of prevention and treatment of high blood pressure with special attention paid to the role of nitric oxide and oxidative stress in regulation of blood pressure and vascular functions. Significant part of her research is focused on the vascular effects of various natural substances in prevention and treatment of hypertension. The most recent studies are focused on the research of the role of NRF2- activator dimethyl fumarate (DMF) in the cardiovascular system. She is the author of ~120 peer-reviewed publications in extenso with more than 2000 citations.
Dual Effect of Disulfiram on gene expressions of Ferroptosis Regulator NRF2 and NRF2 Target genes in Multiple Myeloma
Caglar Arkan, Gamze Bozkaya, Yakuphan Baykan and Dilara Akcora-Yildiz
Department of Biology, Art&Science Faculty, Mehmet Akif Ersoy University, Burdur, Turkey
Email: dilaraakcora@mehmetakif.edu.tr
Multiple myeloma (MM), the second most prevalent type of blood cancer globally, is caused by the excessive proliferation of immunoglobulin-producing plasma cells in the bone marrow. Although recent therapeutic advances in the treatment of MM have been highly effective in increasing the survival of patients with MM, MM remains incurable, as most patients inevitably relapse and become resistant to current therapies. Therefore, there is a need to develop new agents that target different molecular pathways that drive malignancy in MM.
Increasing evidence suggested that ferroptosis, a regular non-apoptotic form of cell death characterized by increased iron-dependent intracellular reactive oxygen species (ROS) production and thus lipid peroxidation, is involved in MM’s development and treatment response. The transcription factor nuclear factor erythroid derived-2-like 2 (NRF2), the central regulator of ROS detoxification and implied as a promoter of drug resistance in MM cells, plays a vital role in ferroptosis. Recently, disulfiram (DSF), which has been widely used for years in the US Food and Drug Administration (FDA) approved treatment of alcoholism and known to exhibit anticancer effects in various types of cancer, including MM, has been shown to be a ferroptosis inducer. However, DSF displays either as an activator or inactivator of NRF2 in different pathologies. Therefore, in the context of MM, we investigated the effect of DSF with or without liproxstatin-1 (Lip1), a ferroptosis inhibitor, on gene expression of NRF2 and NRF2 target genes such as heme-oxygenase-1 (HO-1), glutathione peroxidase-4 (GPX4), spermidine/spermine N1-acetyltransferase 1(SAT1) in p53 wild-type and null MM cell lines. We found that DSF treatment led to ferroptotic cell death in MM cell lines. We then observed that DSF treatment caused a significant decrease in GPX4 and SAT1 mRNA expression in both p53 wild-type and mutant MM cell lines. Interestingly, DSF treatment substantially upregulated the expression of NRF2 and HO
1 in NCI H929 cells in which p53 wild type (p53+/+) and significantly downregulated in RPMI 8226 cells in which p53 is null (p53-/-) and the addition of Lip-1 to DSF treatment could not increase their expression. These results suggest that DSF acts as an activator or inactivator of NRF2, dependent on p53 expression. Further studies are also required using shRNA technology in the treatment of sensitive and resistant myeloma cell lines and in primary myeloma cells from MM patients. This work was based on a Master of Science Thesis and supported by Burdur Mehmet Akif Ersoy University Scientific Research Projects Unit Under Project number 0684-YL-20.

Dilara Akcora Yildiz is an Assistant Professor at Biology Department, Mehmet Akif Ersoy
University. She graduated from the Biology Department, Faculty of Science, Ege University, Turkey in 2004. She received her master’s degree from the Medical Biology Department, Faculty of Medicine, Ankara University, Turkey in 2007 and studied the effect of T315I, E255K and M351T mutations in imatinib resistance in chronic myeloid leukaemia patients. In the same year she was awarded with a Postgraduate Education Scholarship in Australia by the Ministry of National Education of Turkey (MEB) (2008-2012). She then earned her Ph.D. degree in intestinal stem cell biology at Department of Pathology at The University of Melbourne in 2012. During her doctoral studies, she characterized the role of colony stimulating factor 1 receptor-ligand pair (Cfms/CSF1) and granulocyte macrophage colony stimulating factor (GM-CSF) in intestinal biology. She was the principal investigator of a research projects which were supported by The Scientific And Technological Research Council Of Turkey (TUBITAK). She is currently working as an investigator in other projects focused on multiple myeloma biology, brain tumors, cancer stem cells and antibody production. Dr. Akcora Yildiz was a participant at 9th HOPE Meeting with Nobel Laureates in 2017.
- 13:10 – 14:10 Lunch | Poster Viewing | Group Photo
- 14:10 – 16:10 Scientific Session V: NRF2 from Pathomechanisms to Therapeutics Chairs: Nesrin Kartal Ozer, Turkey, and Theo Zacharis, Greece
Targeting the NRF2/beta-TrCP axis in liver inflammation
Raquel Fernández-Ginés, Ana I. Rojo, and Antonio Cuadrado
Instituto de Investigaciones Biomédicas “Alberto Sols” UAM-CSIC, Instituto de Investigación Sanitaria La Paz (IdiPaz) and Department of Biochemistry, Faculty of Medicine, Autonomous University of Madrid, Madrid, Spain. Centro de Investigación Biomédica en Red Sobre Enfermedades Neurodegenerativas (CIBERNED), ISCIII, Madrid, Spain
Email: antonio.cuadrado@uam.es
It is widely accepted that activating the transcription factor NRF2 will blast the physiological anti-inflammatory mechanisms, which will help combat pathologic inflammation. Much effort is being put in inhibiting the main NRF2 repressor, KEAP1, with either electrophilic small molecules or disrupters of the KEAP1/NRF2 interaction. However, targeting β-TrCP, the non-canonical repressor of NRF2, has not been considered yet. After in silico screening of ∼1 million compounds, we now describe a novel small molecule, PHAR, that selectively inhibits the interaction between β-TrCP and the phosphodegron in transcription factor NRF2. PHAR upregulates NRF2-target genes such as Hmox1, Nqo1, Gclc, Gclm and Aox1, in a KEAP1-independent, but β-TrCP dependent manner, breaks the β-TrCP/NRF2 interaction in the cell nucleus, and inhibits the β-TrCP-mediated in vitro ubiquitination of NRF2. PHAR attenuates hydrogen peroxide induced oxidative stress and, in lipopolysaccharide-treated macrophages, it downregulates the expression of inflammatory genes Il1b, Il6, Cox2, Nos2. In mice, PHAR selectively targets the liver and greatly attenuates LPS-induced liver inflammation as indicated by a reduction in the gene expression of the inflammatory cytokines Il1b, TNf, and Il6, and in F4/80- stained liver resident macrophages. Thus, PHAR offers a still unexplored alternative to current NRF2 activators by acting as a β-TrCP/NRF2 interaction inhibitor that may have a therapeutic value against undesirable inflammation.

Antonio Cuadrado is a full professor of Biochemistry and Molecular Biology at the Department of Biochemistry, Medical School, Autonomous University of Madrid. He obtained his PhD degree in Biology in 1985 and enjoyed several postdoctoral stays in the National Cancer Institute-NIH with the help of Fulbright and Fogarty fellowships. He established his independent laboratory as Professor of Biochemistry in 1997 with a main interest on the study of molecular mechanisms involved in initiation and progression of chronic diseases. For the past years his main lane of research has been the validation of transcription factor NRF2, master regulator of cellular homeostasis as a new therapeutic target in chronic diseases with particular emphasis in neurodegenerative diseases (Alzheimer and Parkinson) and in fatty liver diseases. His current interest is the development of new NRF2-modulating drugs. Dr. Cuadrado has published over 160 primary and review articles, of which more than 80 are related to the role of NRF2 in physiological and pathological responses to disease.
The role of NRF2 signaling pathway in the early phase of liver steatosis: a high‐fat diet‐fed rat model supplemented with liquid fructose
Benedetta Di Veroli1, Roger Bentanachs2, Marta Alegret2, Elisabetta Profumo1, Luciano Saso3, Juan Carlos Laguna2, Brigitta Buttari1
1 Department of Cardiovascular and Endocrine-metabolic Diseases and Aging, Istituto Superiore di Sanità, 00161 Rome, Italy
2 Department of Pharmacology, Toxicology and Therapeutic Chemistry, School of Pharmacy and Food Science; Institute of Biomedicine, University of Barcelona, Barcelona 08028 Spain; Spanish Biomedical Research Centre in Physiopathology of Obesity and Nutrition (CIBEROBN), Instituto de Salud Carlos III (ISCIII), 28029, Madrid Spain
3 Department of Physiology and Pharmacology, Sapienza University of Rome, Vittorio Erspamer, 00161 Rome, Italy
Email: brigitta.buttari@iss.it
Nonalcoholic fatty liver disease (NAFLD) is a metabolic disorder characterized by excessive fat accumulation. Insulin resistance, increased lipogenesis, gluconeogenesis, inflammation, and oxidative stress are mechanistically linked to dysfunction of the antioxidant response controlled by the transcription factor nuclear factor E2-related factor 2 (NRF2). The pharmacologic activation of NRF2 reverses nonalcoholic steatohepatitis (NASH) in mouse models, however to date there are no treatments for NAFLD/NASH other than lifestyle changes or liver transplantation. Although the NRF2 signaling pathway is well understood in high fat or diabetic models, information is lacking about the early phase of steatosis. The aim of this study is to delineate the role of the antioxidant response in the initial phase of NAFLD spectrum in a dietary rat model of fatty liver without inflammation. In three months, female rats fed a high-fat diet without cholesterol, supplemented with a 10% w/v fructose solution as beverage (HFHFr), developed fatty liver independently of other metabolic disturbances. To compare the antioxidant response of rats fed the HFHFr or a standard chow (CT) diet, the expression of oxidative stress regulator genes such as NRF2, heme oxygenase-1 (HO-1) and NAD(P)H Quinone Dehydrogenase 1 (NQO1) in liver was evaluated by quantitative real-time PCR and western blot. Although the addition of liquid fructose to the high fat diet is determinant to produce liver steatosis, none of the groups showed marked inflammation or oxidative stress. In fact, HO-1 protein levels were reduced, whereas NQO1 protein levels were upregulated in HFHFr group compared to the CT, and a slight imbalance of NRF2/Keap1 ratio was observed. These findings lead us to conclude that in our fructose-induced steatosis model the early intrahepatic oxidative stress is counteracted by NRF2-induced antioxidants thus preventing radical formation and tissue damage. Consequently, NRF2 activators should be important in the prevention and treatment of NAFLD/NASH.

Brigitta Buttari is a Researcher at Istituto Superiore di Sanità (ISS- Italian National Institute of Health), Italy. She was awarded Specialty in Applied Biotechnology in 2003 and PhD in Medical Microbiology and Immunology in 2008. Her main research interest is the study of cellular and molecular mechanisms involved in initiation and progression of inflammatory chronic diseases. A special research interest include the investigation of the natural compounds as modulators of oxidative stress and cellular processes to prevent cell damage and to suppress inflammation through modulation of the Keap1-NRF2 signaling pathway.
Thiosulfate Sulfurtransferase Deficiency Promotes Oxidative Distress and Aberrant NRF2 Function in the Brain Tissue
Yang Luo1,2, Laurent Chatre3, Shaden Melhem4, Zayana M. Al-Dahmani1, Natalie Z.M. Homer4, Martin Feelisch5, Matthew Groves1, Nicholas M. Morton4, Amalia Dolga1, Harry van Goor2
1 University of Groningen, the Netherlands, 2University Medical Center Groningen, the Netherlands, 3Université de Caen Normandie, France, 4University of Edinburgh, United Kingdom, 5University of Southampton, United Kingdom
Email: h.van.goor@umcg.nl
Background Thiosulfate sulfurtransferase (TST, EC 2.8.1.1) was discovered as a rhodanese that detoxifies cyanide with thiosulfate as substrate. Its characteristics relate to sulfide metabolism, antioxidant defense and mitochondrial function, which are important protective biological processes oxidative distress. While TST has been described to play an important role in liver and colon tissue, its potential involvement in the brain function remains obscure.
Aims We study the effect of TST deficiency in mice on the redox balance in the cerebral cortex, the area of extensive neuronal activities and thereby sensitive to oxidative distress.
Methods C57BL/6J and Tst-/- 6-month old mice were used. Cortices were evaluated for antioxidant enzymes protein expression and activity levels via immunoblot and activity assays. The OXPHOS proteins and mitochondrial respiration were measured by immunoblot and high resolution respirometry. Fluorescent probes were utilized to measure ROS and RSS. Total ATP level was detected by luminescence assay
Results Tst-/- mice had a striking 20-fold and 475-fold elevation in the trunk plasma and urine of thiosulfate compared to WT. The cortex of the Tst -/- mice contains significantly decreased level of H2S and increased H2Sn. We found a dysregulated pathway downstream of H2S, as evidenced by decreased GSH/GSSG ratio and GPX4 protein expression. We also observed an increase in total ATP level in Tst -/- cortices. High
resolution respirometry showed that Tst-/- cortices have an increase in mitochondrial complex IV activity, suggesting mitochondrial fitness. H2O2 and O2-, as products of OXPHOS, were found increased in Tst-/- cortical brain samples. Finally, we found a significant decrease in SOD activity and an increase in catalase activity in Tst-/- cortexes compared with WT. Nrf2 protein and Keap1 protein displayed disrupted expression levels in Tst-/- mice brain samples compared to WT.
Conclusion We provide a first glimpse into the molecular and metabolic changes of TST deficiency in the brain and suggest that pathophysiological conditions associated with aberrant TST expression and/or activity renders neurons more susceptible to oxidative stress-related malfunction via NRF2/Keap1 associated mechanisms.

Harry van Goor is a fundamental researcher and a translational scientist who completed a PhD program at the University of Groningen and a post-doctoral fellowship at the Penn State University, Hershey, PA, USA. He is currently involved in various projects focusing mainly on the role of the reactive species interactome (ROS, NO and H2S) and NRF2 in COVID-19, aging, neurodegeneration, CV disease and the metabolic syndrome. His research group consists of MD/PhD students and PhD students from different nationalities and backgrounds. Harry van Goor has received several research grants from the Dutch Kidney Foundation. He was past President of the International society of Antioxidants, Nutrition and Health, Associate Editor of Renal Pharmacology and Management team member of the Cost Action on “Bench to bedside transition for pharmacological regulation of NRF2 in non-communicable diseases (BenBedPhar)” representing the Netherlands.
Nutrient-dependent alterations of stress responses in the brain: deciphering the role of NRF-2
Fabio Di Domenico, Chiara Lanzillotta, Francesca Prestia, Marzia Perluigi
Department of Biochemical Science, Sapienza University of Rome
Email: fabio.didomenico@uniroma1.it
Alterations of stress responses and brain dys-metabolism are emerging as strong contributors to cognitive deterioration, however, no data concerning their initiation, progression and mutual crosstalk have been produced yet. We demonstrated in Down syndrome (DS) and Alzheimer Disease (AD) that the aberrant activation of the PERK pathway of the unfolded protein response (UPR) precede the appearance of frank neuropathological hallmarks and is associated with the depletion of NRF-2-related antioxidant response. In the same brain specimens, we showed that metabolic defect occurs in concomitance to faulty stress responses and are linked with redox imbalance and cognitive decline. In addition, we observed that the pharmacological inhibition of PERK, in DS mice, promoted the metabolic reprogramming of the brain and rescued the PERK/NRF-2 through a mechanism involving the modulation of PI3K activity, a downstream effector of insulin signalling. Recently to better elucidate the relationship between those events in the progression of neurodegenerative mechanisms, we subjected primary brain and peripheral cells to nutrient overload, demonstrating that aberrant metabolic stimuli were able to initiate and/or aggravate the de-regulation of PERK/NRF-2 axis mimicking the impairments observed in AD and DS. As well, by pharmacologically targeting the PERK pathway we were able to ameliorate brain flaws associated to overnutrition by improving protein homeostasis and NRF-2 signalling. Our results suggest that metabolic defects occurring in AD-like pathologies (but also in normal population as effect of unbalanced nutrition) exacerbate the failure to regulate stress responses promoting NRF-2 signal depletion, redox imbalance and neurodegeneration.

Fabio Di Domenico is Full professor of Biochemistry at Sapienza University of Rome. He obtained his PhD degree in Biochemistry in 2009. Before gaining his current position, he performed his research under the supervision of Prof. Butterfield at University of Kentucky, where he has been involved in the application of redox proteomics studies. His research is currently focused in understanding the mechanisms that associates increased redox imbalance, defective proteostasis and brain dys-metabolism in the development of Alzheimer-like dementia. Collected data from his laboratory postulate that aberrant proteostasis, observed in both Alzheimer and Down syndrome patients, is strictly associated with the increase of oxidative damage as result of compromised antioxidant response and faulty protein
degradative systems. Recently, his studies revealed that the chronic induction of the unfolded protein response and its aberrant relationship with Nrf2 response hold a prominent role in the development of dementia in the brain from Alzheimer and Down Syndrome patients.
PAS GRAS – a unique interdisciplinary project to revert obesity throughout life
Paulo Matafome1,2,3*; Eugénia Carvalho3,4,5, John Jones3,4,5; Anabela Marisa Azul4,5; Paulo Oliveira3,4,5
1 Polytechnic University of Coimbra, Coimbra Health School, Coimbra, Portugal
2 Coimbra Institute for Clinical and Biomedical Research (iCBR), Center for Innovative Biomedicine and Biotechnology (CIBB) and Faculdade de Medicina, Universidade de Coimbra, Coimbra, Portugal 3 Centro Académico-Clínico de Coimbra (CACC), Coimbra, Portugal
4 CNC—Center for Neuroscience and Cell Biology, CIBB, University of Coimbra, Coimbra, Portugal 5IIIUC—Institute for Interdisciplinary Research, University of Coimbra, Coimbra, Portugal
Email: paulo.matafome@estesc.ipc.pt
The growing burden of obesity worldwide dictates a cross-disciplinary strategy involving biomedicine, life and social sciences to identify in depth its causes, and design reliable and effective prevention and treatment interventions. PAS GRAS is centered on overweight/obese children, adolescents, and young adults with the main objective of reversing obesity and preventing later-life comorbidities. PAS GRAS will fill critical gaps in obesity diagnosis and prognosis, early lifestyle and pharmacological intervention, obesity awareness and food/nutrition literacy, and provide a blueprint for a healthier population.
One of the dietary approaches recommended to combat overweight and obesity is the Mediterranean Diet (MeD), recognized by the United Nations Educational, Scientific and Cultural Organization (UNESCO) as an “Intangible Cultural Heritage of Urgent Safeguarding”. Originating from the food culture of ancient civilizations established around the Mediterranean Basin, the MeD includes high consumption of specific vegetables, like plants in the family Brassicaceae rich in sulforaphane (SNF), fruits, grains, moderate consumption of fish, seafood, and olive oil. The MeD is balanced with low intake of red meat and other meat products and is both healthy and environmentally sustainable. Although the positive effects of the MeD on decreasing mortality, cardiometabolic disease and cancer have been recognized scientifically, most likely through its antioxidant, anti-inflammatory, anti-thrombotic and lipid lowering effects, the biological mechanisms and targets for the activity of MeD components are still not understood. PAS GRAS will conduct pre-clinical and clinical studies to identify basic mechanisms responsible for the protective effects of bioactive ingredients to counteract obesity, namely the MED-derived compounds able to target fatty acid oxidation and NRF2, including SFN. With this new evidence-based approach, PAS GRAS will promote the Mediterranean Diet as a tool for a healthier and sustainable future.
Funding: European’ Union Horizon Europe under the agreement No 101087416

Paulo Matafome holds a PhD in Biomedical Sciences since 2012 (Faculty of Medicine, University of Coimbra). He is a Professor in the Polytecnic University of Coimbra and a Integrated member of CIBB (research center of University of Coimbra). He has published 91 research articles and 4 book chapters (>2000 citation, h=25). He has supervised or co-supervised 12 Ph.D. students (4 concluded), 18 MSc students, and 21 BSc Students. He has participated in 19 Research Projects and is one of the co-coordinators of PAS GRAS (Horizon Europe). Paulo Matafome has collaborations with the Departments of Surgery and Endocrinology of the University Hospital Center of Coimbra, with Brazilian, Spanish and Portuguese groups in the field of metabolic programming, and with the Institute of Health Applied Nuclear Sciences, developing new state-of-the-art bioimaging biomarkers (human and animal PET and MRI) for metabolic diseases complications. He has studied the neuroendocrine mechanism controlling the gut-adipose tissue crosstalk. He has access to human blood and adipose tissue samples, animal models of diabetes (Goto-kakizaki colony) and his lab has developed methods to evaluate angiogenesis and adipose tissue blood flow ex vivo and in vivo. He has studied several new antioxidant molecules in diabetic models in order to prevent vascular decline in adipose tissue.
NRF2 clinical trial and patent landscape
Marisa Antunes, Fernando Antunes
Centro de Química Estrutural, Institute of Molecular Sciences, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa, Lisboa, Portugal
Email: fc55119@alunos.fc.ul.pt
Nrf2 (Nuclear factor-erythroid factor 2-related factor 2) is a transcription factor encoded by the NFE2L2 gene in humans. This factor is one of the major regulators of antioxidant enzymes and cytoprotective genes, and in some diseases the deficient production of this factor increases oxidative damage, inflammatory processes, and cellular apoptosis. Two databases containing information about clinical trials and patents related to Nrf2 will be described to facilitate the economic utilization of the outcomes generated by BenBedPhar.

Marisa Antunes (Master’s in Biochemestry). Research fellow at Centro de Química
Estrural (CQE) of the Faculty of Sciences of the University of Lisbon. Marisa was a
biomedical laboratory scientist at the Germano de Sousa Group – Center for
Laboratory Medicine, and now she is a research fellow at the CQE. She is
developing the following projects as a researcher: (1) Microcalorimetry and
fluorescence nanothermometry in early detection of immune T cell exhaustion; and
a systematic review (2) Clinical trials assessing Nrf2/ARE pathway as a therapeutic
target.
- 16:10 – 16:30 Coffee Break | Poster Viewing
- 16:30 – 17:20 Guided Poster Sessions (parallel sessions, 3 min presentation and 2 min discussion) | Session A Chairs: Irina Milisav, Slovenia, and Isabel Lastres-Becker, Spain
Using CRISPR/Cas9 to knock-out the NRF2 gene in retinal pigment epithelium cells
Ana S Falcão, Margarida Pedro, Pedro Antas, Sandra Tenreiro and Miguel C. Seabra
iNOVA4Health, NOVA Medical School|Faculdade de Ciências Médicas, NMS|FCM, Universidade Nova de Lisboa; 1169-056 Lisboa, Portugal
Email: ana.falcao@nms.unl.pt
Age-related macular degeneration (AMD) is currently an incurable and widespread disease that causes blindness. One of the main pathologic features of AMD is the accumulation of autofluorescence (lipofuscin) in retinal pigment epithelium (RPE) cells leading to their degeneration and death. Among the cell damaging events is photodamage of lipofuscin which may induce oxidative stress (OS). In fact, the retina is especially prone to OS due to high oxygen pressure and exposure to UV and blue light, promoting reactive oxygen species generation. NRF2/NFE2L2, as the master regulator of the anti-OS response, has recently been implicated in human AMD and investigated as a therapeutic target. The activity of NRF2 in the RPE of aged mice has been found to be impaired, indicating that the aging RPE has a limited capacity to respond to perturbations in OS levels, and suggesting that an impaired anti-OS response by aging RPE after an oxidative stimulus could induce RPE dysfunction that may contribute to the development of AMD.
We have developed a model that recapitulates some AMD features in vitro, where feeding human RPE monolayers in culture with porcine photoreceptor outer segments (POS) leads to accumulation of autofluorescent granules similar to lipofuscin in vivo, mimicking RPE cellular stress and disease. Our preliminary results showed that overexpression of the transcriptional regulator NRF2, as well as treatment with NRF2 activators, can prevent the formation and/or resolve POS-derived autofluorescent granules, suggesting that this pathway and the proteins it controls are important for autofluorescent granule formation.
To further dissect the role of NRF2 in our in vitro model of AMD, we developed a stable NRF2 KO using CRISPR/Cas9 technology in a RPE cell line. After selection of the best-fitted single guide RNA to target the NFE2L2 gene, generation of knock-outs in ARPE-19 cells was achieved by electroporation. The gene-edited region of the NFE2L2 gene was confirmed by Sanger sequencing.
Overall, these NRF2 KO cells will be a valuable tool when exploring the role of NRF2 as a therapeutic target in AMD, to dissect direct versus indirect mechanisms of action of involved pathways and drug activities. Moreover, this will be a new tool available for collaborators in the context of BenBedPhar COST action.
Project funded by “La Caixa Foundation” (NASCENT HR22-00569)

Ana Sofia Falcão holds a PhD in Pharmacy since 2006 and she is currently working as a post doctoral researcher at the Molecular Mechanisms of Disease Lab, at Nova Medical School (NMS), from Universidade Nova de Lisboa. At NMS, she became interested in exploring innovative therapeutic approaches for neurodegenerative disorders or other age-related diseases, such as AMD. She is focused on in vitro systems to study retinal pigment epithelial cells that will be used for fundamental studies as well as for potential translational applications to treat retinal disorders.
Analysis of expression patterns of NRF2 targets in association with TP53 status and inducers in MCF7 breast cancer cells
Abdul Moiz Aftab1, Ayse Gokce Keskus2, Merve Vural2, Cagdas Yanyatan1, Burcin Irem Arici1, Yagmur Oyku Carus1, Onur Cizmecioglu1, Ozlen Konu1,2
1 Molecular Biology and Genetics department, Bilkent University, Ankara, Turkey
2 Neuroscience Program, Bilkent University, Ankara, Turkey
NRF2 (Nuclear factor erythroid 2-related factor 2) is a transcription factor that plays an important role in cellular defence against oxidative stress and inflammation. NRF2 has also been implicated in various other pathologies, including neurodegenerative diseases, metabolic disorders, and cancer. TP53 is the most commonly mutated gene in multiple cancers including breast carcinoma and its targets could be upregulated by depletion of CHRNA5, a cholinergic receptor, or doxorubicin, a topoisomerase inhibitor used as an anticancer agent. However, the association between the TP53 status, CHRNA5 levels and/or doxorubicin and the expression of NRF2 target genes is largely unknown. Here, we performed RNA sequencing using MCF7 cells treated with siRNAs against CHRNA5, TP53 or both as well as wildtype TP53 over-expressing and isogenic heterozygous mutant TP53 expressing MCF7 cells. We obtained the NRF2 target lists from different online sources and generated heatmaps and treatment -gene bipartite co expression networks to identify the NRF2 targets influenced by TP53 expression or mutation status in the presence or depletion of CHRNA5. Interestingly we observed that HMOX1, a known mutant TP53 repressed target, was upregulated significantly in mutant cell lines when treated with the siCHRNA5. Moreover, we tested whether addition of doxorubicin, another TP53 inducer, modulated further the targets specific to NRF2. For example, TXN was upregulated in wildtype TP53 over-expressing siCHRNA5 treated cells but downregulated when doxorubicin was present together with the siCHRNA5. Our pipeline can be applied to other datasets consisting of combinatorial treatments to better understand the mechanisms governing genotypic and drug induced variability in NRF2 signalling in different cancers. This study has been funded by a research grant from TUBITAK (Project no. 219Z029).
Aquaporins in the Spotlight: Unraveling Their Interaction with NRF2 in Breast Cancer
Monika Mlinarić1, Ana Josipa Jerončić2, Ivan Lučić1, Lidija Milković1, Ana Čipak Gašparović1
1 Ruđer Bošković Institute, Zagreb, Croatia
2 Faculty of Science, University of Zagreb, Zagreb, Croatia
Email: acipak@irb.hr
Aquaporins (AQPs) are integral membrane channels responsible for maintaining cellular water balance and facilitating the transport of various molecules, including hydrogen peroxide. Beyond their well-established role in water transport, AQPs have a significant role in the context of cancer due to their involvement in tumor progression and metastasis, with their expression often dysregulated. Understanding the delicate regulation of cellular oxidative stress and the role of AQPs in this process is important in defining their impact on breast cancer progression. Moreover, studying the potential interaction between AQPs and NRF2, a master regulator of the antioxidant response, can offer valuable insights into how cells manage and control oxidative stress. In this study, we aimed to explore the influence of NRF2 activation using sulforaphane and NRF2 inhibition with ML385 on the expression of AQP3 and AQP5 in different breast cancer cell lines and a non-tumorigenic breast epithelial cell line. We evaluated cell viability and proliferation following the treatments, and selected concentrations that effectively modulate NRF2 expression. We further examined the impact of NRF2 modulation on AQP expression at both the transcriptional and protein levels. Additionally, we assessed AQP activity in terms of hydrogen peroxide transport to gain a complete understanding of the effects of NRF2 on AQPs. Our preliminary findings suggest a potential interplay between NRF2 and AQPs, as changes in AQP expression and activity were observed following NRF2 modulation, with cell type-specific variations. However, to fully understand the intricate cross-talk between AQPs and NRF2, further investigation is required to elucidate the precise underlying mechanisms.

Monika Mlinarić is a PhD student working in the Laboratory for Oxidative Stress, Division of Molecular Medicine at Ruđer Bošković Institute. Under the guidance of Dr. Ana Čipak Gašparović, her research focuses on “Elucidating the role of aquaporins 3 and 5 in the development of breast cancer resistance to oxidative stress (AquaBCaRe).” Her PhD investigation involves studying the interaction between NRF2 and aquaporins, as well as their response to chronic oxidative stress conditions.
Nrf2 down-regulation mediates pro-inflammatory effects of novel synthetic AhR ligands
Ivan Koprivica1, Natalija Jonić1, Christos Chatzigiannis2, Antonis Tsiailanis2, Andreas G. Tzakos2, Ivana Stojanović1
1Institute for Biological Research “Siniša Stanković” – National Institute of the Republic of Serbia, Department of Immunology, University of Belgrade, Belgrade, Serbia
2 Section of Organic Chemistry & Biochemistry, Department of Chemistry, University of Ioannina, Ioannina, Greece
Email: ivan.koprivica@yahoo.com
Aryl hydrocarbon receptor (AhR) and nuclear factor erythroid 2-related factor 2 (Nrf2) are transcription factors involved in the regulation of drug-metabolizing enzymes. Moreover, both of them can modulate the immune response. AhR activation can lead to the activation or inhibition of specific immune cells, especially at barrier tissues such as skin, lungs, gut-associated lymphoid tissue, etc. Nrf2 was also shown to play a role in the anti
inflammatory process by inhibiting the recruitment of inflammatory cells and regulating anti-inflammatory gene expression. Nrf2 gene transcription can be directly modulated by AhR activation, as the Nrf2 promoter possesses three xenobiotic response element-like elements that were shown to be able to bind AhR in response to a known Ahr agonist TCDD.
In this study, we explored the effect of newly synthetized AhR agonists (indole-based derivatives) termed C46 and B19 on mouse macrophage differentiation. Peritoneal cells were incubated with 1.5 µM of AhR ligands for 24 h, after which the proinflammatory M1 (F4/80+CD40+) and anti-inflammatory M2 (F4/80+CD206+) macrophage phenotype was determined by flow cytometry. The results indicate that both compounds push macrophages towards a more inflammatory state, as C46 tripled the M1/M2 ratio in culture, while B19 doubled it, compared to the DMSO (0.005% v/v) control. Additionally, both mRNA and protein expression of cytochrome P450 1A1 (CYP1A1), commonly used as an indicator of AhR activation, were also increased by C46 and B19. Finally, western blot analysis showed that both of the tested AhR ligands downregulated the protein expression of Nrf2 within the treated cells.
These results suggest that AhR activation and subsequent Nrf2 down-regulation by the newly synthesized AhR agonists C46 and B19 boosted the proinflammatory phenotype of mouse peritoneal macrophages.

Ivan Koprivica is a Research Associate at the Department of Immunology, Institute for Biological Research “Siniša Stanković” – National Institute of the Republic of Serbia, University of Belgrade, Serbia, where he has been a part of the Diabetology Research Laboratory since 2016. His main research interest is focused on identifying cellular and molecular mechanisms responsible for Type 1 Diabetes (T1D) development in mice, as well as pharmacological modulation of experimental T1D, using a range of bioactive compounds in order to treat T1D autoimmunity. Recently, his research interests have also included investigating the role of gut immune cells in T1D development, as well as exploring the immunomodulatory potential of several novel synthesized AhR ligands.
Deregulation of multiple mechanisms shape the onset of LAMA2- congenital muscular dystrophy
Susana G Martins1, Vanessa Ribeiro1, Catarina Melo1, Cláudia Paulino-Cavaco1, Sharadha Naidu Dayalan2, Inês Fonseca1, Bérénice Saget1, Albena Dinkova-Kostova2, Solveig Thorsteinsdóttir1*, Ana Rita Carlos1*
1 Centre for Ecology, Evolution and Environmental Change (cE3c), Faculty of Sciences of the University of Lisbon (FCUL) & Global Change and Sustainability Institute (CHANGE), Portugal
2Jacqui Wood Cancer Centre, School of Medicine, University of Dundee, Scotland
* Co-senior authorship
Email: sgmartins@ciencias.ulisboa.pt
Muscular dystrophies are linked to mutations in genes encoding extracellular matrix-related components and are characterized by muscle weakness, chronic inflammation and increased oxidative stress. Therefore, NRF2 activation has been explored as a potential target for treatment. A such example is LAMA2-congenital muscular dystrophy (LAMA2-CMD), triggered by mutations in LAMA2 gene. Using the dyW mouse model for LAMA2-
CMD, we have previously shown that LAMA2-CMD starts in utero with a reduction in the pool of muscle stem cells (MuSCs), a dramatic reduction in the number of differentiated myoblasts and impaired muscle growth. To explore the cellular and molecular mechanisms involved in this fetal muscle phenotype, we generated a Lama2- deficient C2C12 myoblast cell line. Our data revealed that Lama2-deficient C2C12 cells exhibit a decreased proliferation rate in vitro, possibly due to a cell cycle arrest in G0/G1 phase, and increased levels of DNA damage. Lama2-deficient C2C12 cells also showed increased levels of HO-1 protein and decreased levels of reduced glutathione and the transporter Slc7a11, all signs of increases oxidative stress, a condition known to influence cell cycle progression. In addition, Lama2-deficient C2C12 myoblast cells also showed reduced levels of MYF5, a myogenic factor, in the nucleus and impaired differentiation. To better understand the mechanisms altered in Lama2-deficiency, we performed a RNAseq analysis of fetal muscle fibers. This analysis revealed important changes in genes linked to cell cycle, oxidative stress, plasma membrane signaling and cytoskeleton structure. In fact, our validation data revealed that skeletal muscles from Lama2-deficient fetuses display altered expression of genes linked to cell cycle regulation, increased DNA damage and decreased levels of NQO1. These results are particularly important since they contribute for a better understanding of the first stages of LAMA2-CMD onset, which is essential to design therapies that specifically target the primary events underlying this incurable, and often lethal, disease.

Susana G Martins: I am a PhD Student at the Development and Evolutionary Morphogenesis Laboratory at cE3c (Faculty of Sciences, Lisbon University) under the supervision of Drs. Ana Rita Carlos and Sólveig Thorsteinsdóttir, with a MSc in Molecular Genetics and Biomedicine from FCT/UNL. The project I have been working on contributed to 2 oral presentations (one awarded with the prize for best short talk) and 12 posters presented in national and international meetings. Additionally, I actively contributed to the writing of two review articles, “Linking Oxidative Stress and DNA Damage to Changes in the Expression of Extracellular Matrix Components” and “NFIXing Cancer: The Role of NFIX in Oxidative Stress Response and Cell Fate”. I am also directly involved in mentoring master and undergraduate students and, last year, I was one of the winners of the Participatory Budget of cE3c. Recently, I received a grant for a Short-Term Scientific Mission within BenBedPhar Cost Action.
Activation of Nrf2 pathway and related antioxidant proteins in INS-1 cells treated with Hypericum perforatum transgenic roots extracts
Biljana Miova1, Nadezda Apostolova2,3,4, Juan V. Esplugues2,3,4, Suzana Dinevska Kjovkarovska1, Elena Rafailovska1
1 Department of Experimental Physiology and Biochemistry, Institute of Biology, Faculty of Natural Sciences and Mathematics, “Ss Cyril and Methodius” University in Skopje, R.North Macedonia 2 Department of Pharmacology, Faculty of Medicine, University of Valencia, Valencia, Spain 3 CIBERehd (National Network of Biomedical Research on Hepatic and Digestive Diseases), Spain 4 FISABIO (Foundation for the Promotion of Health and Biomedical Research in the Valencian Region), University Hospital Doctor Peset, Valencia, Spain.
Email: bmiova@pmf.ukim.mk; bmiova@yahoo.com
Genetic transformation of H. perforatum with A. rhizogenes leads to the establishment of H. perforatum transgenic culture (Hairy Roots, HR) which accumulated significant quantities of various xanthones. Xanthones are distinguished by a wide range of biological activities of which the antioxidant and antidiabetic are more pronounced and shown in animal models, humans and in vitro. They are promoted as a potential antidiabetic therapy by stimulating β-cell regeneration and preventing functional β-cell damage. The aim of this research is to evaluate the effects of different HR treatments (HR1-methanolic extract, HR2 – ethyl acetate exctact and HR3 – hexane extraxt) and concentration (200, 100, 50, 25 and 12.5 µg/ml) on the cell viability of INS-1 pancreatic cell line. Also, gene expression (by RT-qPCR ) of several genes related to Nrf2 signaling pathway was assessed (100 and 50 µg/ml). Mangiferin and metformin were employed as a standard and a positive control, respectively.
MTT test showed that all HR-extracts, mangiferin and metformin did not reduce cell viability except that the highest concentration (200 µg/ml) displays a certain degree of cytotoxicity.
HR1 extract increased mRNA levels of kelch-like ECH-associated protein 1 (KEAP1) and heme-oxygenase 1 (HO-1); HR2 extract did not caused changes in gene expression of neither of the studied genes, while HR3 extract caused significant up-regulation of gene expression of NFR2, thioredoxin (TXN1) and glutathione reductase (GSR). Mangiferin caused significant up-regulation of NFR2, GSR and TXN1, while metformin caused significant up-regulation of gene expression NFR2, KEAP1, HO-1, GSR and TXN1. In conclusion, HR1 and HR3 extracts could have some beneficial effects by activation of Nrf2 pathway and related antioxidant proteins in cells. Still, the effect of HR3 is more pronounced. The effects of all HR extracts in INS-1cells are comparable to those of mangiferin and metformin.

Biljana Miova is Full professor at the Institute of Biology, Faculty of Natural Sciences and Mathematics, “Ss Cyril and Methodius” University, Skopje, R. North Macedonia. The main interest of her scientific work is Experimental diabetes (treatments with plant extracts or some drugs, exploring the potential utilization in vitro plant cultures for treatment of experimental diabetes); Oxidative stress; Carbohydrate metabolism (key enzymes and substrates); Heat shock proteins, signaling molecules of apoptosis and cell death; High environmental temperature (acute and chronic heat stress, heat preconditioning and cross tolerance). She works with laboratory rats and animal cell cultures.
HYCO-3, an Nrf2 activator that simultaneously releases carbon monoxide (CO), ameliorates type 1 diabetes in a mouse model
Dragica Mićanović1, Goran Stegnjaić1, Neda Nikolovski1, Miljana Momčilović1, Roberta Foresti2, Roberto Motterlini2, Đorđe Miljković1, Tamara Saksida1
1 Department of Immunology, Institute for Biological Research ”Siniša Stanković’’, National Institute of the Republic of Serbia, University of Belgrade, Belgrade, Serbia
2 University Paris Est Créteil, INSERM, IMRB, F-94010, Créteil, France
Email: cvjetica@ibiss.bg.ac.rs
Type 1 diabetes (T1D) is an autoimmune disease that leads to the death of insulin-producing pancreatic β cells. The autoimmune response in T1D becomes chronic as a consequence of regulatory mechanisms unable to counteract this disease. In this study, we evaluated the anti-diabetic potential of a new hybrid compound (HYCO-3) consisting of a CO-releasing molecule conjugated to a fumaric ester derivative that is known to activate the Nrf2 and increase the expression of the CO-producing enzyme heme oxygenase-1. T1D was induced in C57BL/6 mice treated intraperitoneally for 5 consecutive days with multiple low doses of streptozotocin (40 mg/kg BW). HYCO-3 (25 mg/kg BW daily) was administered by oral gavage while the control group received the vehicle (DMSO/sesame oil) for 14 days, starting on the first day of streptozotocin treatment. To evaluate the development of T1D, glycaemia and body mass were measured weekly. At the end of the experiment the pancreas was collected and histochemical analyses for insulin expression were performed. Insulitis, the infiltration of immune cells in the pancreas, was also assessed. We found that treatment with HYCO-3 lowered blood glucose levels compared to the control group in association with a lower insulitis grade and a higher expression of insulin in pancreatic islets. Furthermore, to assess the effect of HYCO
3 on antigen specific response, cells from the draining pancreatic lymph nodes of diabetic animals were stimulated with insulin and HYCO-3 to assess the production of pro-inflammatory markers in cell supernatants. HYCO-3 was able to down-regulate the production of IL-17 in the cultures where antigen specific response was induced with insulin.
Overall, our results show that HYCO-3 ameliorated T1D in C57BL/6 mice by inhibiting the recruitment of immune cells into the pancreas. These results warrant future investigation of cellular and molecular mechanisms by which HYCO-3 acts on immune cells that are of importance in the pathogenesis of T1D.
Tamara Saksida is a Principal Research Fellow at the Department of Immunology at the Institute for Biological Research “Siniša Stanković”, University of Belgrade. Her research interest are pathogenesis and modulation of autoimmune and inflammatory disorders with a focus on connection between gut immune system and distant sites, like pancreas in diabetes or the brain in Alzheimer’s & Parkinson’s disease.
- 16:30 – 17:20 Guided Poster Session | Session B Chairs: Sermin Genc, Turkey, and Christina Morgenstern, Austria
Advances in Blood-Based Biomarkers for Clinical Monitoring of Cardiovascular Diseases -Insights from Computational Modeling
Sumesh Sasidharan
University College Dublin
Email: sumesh4sai@gmail.com
Non-communicable diseases (NCDs) pose a growing global health challenge, and among them, cardiovascular diseases, such as aneurysm and valve regurgitation, remain significant contributors to morbidity and mortality. Recent advancements in computational modeling have opened new avenues for understanding the underlying mechanisms of these conditions, offering insights into the development of blood-based biomarkers for clinical monitoring.
This topic explores the latest research developments in the field of cardiovascular disease assessment. The study leverages computational models to simulate the complex hemodynamic and biomechanical factors associated with aortic aneurysm and valve regurgitation. By integrating patient-specific data with advanced computational techniques, researchers can identify potential blood-based biomarkers that can revolutionize clinical monitoring and management of these conditions.
The areas focused on this study are (i)Hemodynamic simulations and patient-specific modeling for accurate disease characterization. (ii) Identification of novel biomarkers from computational analysis of blood flow dynamics.(iii) Validation and clinical relevance of blood-based biomarkers in aortic aneurysm and valve regurgitation management. and (iv) Future prospects for personalized and precision medicine in cardiovascular disease diagnosis and treatment.
This study explores the cutting-edge research at the intersection of computational modeling and non communicable disease management, as researchers strive to improve the early detection, risk assessment, and overall care for patients with cardiovascular diseases.

Sumesh Sasidharan is Senior Research Engineer at University College Dublin.His primary area of research is the development and application of insilico models in the treatment strategy of various cardiovascular pathological conditions like aneurysms and valvular heart diseases. He is recepient of international awards namely Civis3i Laureate (2023), Marie Curie Individual Fellowship (21-23), Australia Awards Endeavour Fellowship (2018), Elseiver Outstanding Reviewer (2018).
Minocycline attenuates the effects of H2O2 in subcutaneous connective tissue cells
Gülay Sezer
Department of Pharmacology, Faculty of Medicine, University of Erciyes, Kayseri, Turkey
Email: gulayszr@erciyes.edu.tr
Minocycline (Mino) is a tetracycline derivative antibiotic used in clinical practice mainly for the treatment of infectious diseases. Studies have shown that minocycline exerts neuroprotective and anti-inflammatory effects and combats oxidative stress. In this study, Mino-induced protective effects against hydrogen peroxide (H2O2)- induced cell damage and the involvement of nuclear factor erythroid 2-related factor 2 (Nrf2) was investigated. To examine the protective effect of Mino on H2O2-induced cytotoxicity, L929 mouse subcutaneous connective tissue cells were treated with Mino 1 h prior to H2O2 treatment. After incubation with H2O2 for 30 min, medium was decanted and cells were continued to incubation with Mino for 24 h and then MTT cell viability assay was performed. Wound healing assay was performed to investigate whether Mino has any effect on wound closure delay with H2O2. Effect of Mino on H2O2 induced apoptosis was determined by Annexin V labelling. The effect of Mino on H2O2-induced intracellular ROS generation was examined using DCFH-DA assay. qRT-PCR was performed to investigate the expression of Nrf2, Heme oxygenase (Hmox)-1, NAD(P)H quinone dehydrogenase 1 (Nqo1) and Collagen type Ia (Col1a) mRNAs.
Mino did not cause any significant changes in cell viability, but significantly reduced cytotoxicity induced by H2O2 treatment. Wound area was significantly reduced in the Mino-treated cells compared to the untreated control group. Mino pretreatment significantly reduced wound area compared to the H2O2-treated cells. The rate of apoptotic cells increased significantly in H2O2-treated cells, and Mino effectively reversed H2O2-induced apoptosis. ROS levels increased significantly in H2O2-treated cells compared with untreated cells and were significantly inhibited in the presence of Mino. Mino pretreatment significantly increased the mRNA expressions of Nrf2 and Hmox-1 compared to the control and H2O2 groups. Col1a mRNA expression was significantly decreased in the H2O2-treated group, and pretreatment of Mino was not effective in reversing, but Mino administration alone significantly increased the expression level compared to the control group.
The findings of this study suggest that Mino plays a protective role against the cytotoxic, wound closure delaying, apoptotic and oxidative effects of H2O2 probably by reducing ROS production and inducing Nrf2 and Hmox-1 mRNA expressions. Therefore, we propose the further development of minocycline as a cytoprotective and wound-healing agent.

Gülay Sezer Dr. Gülay Sezer is an Associate Professor at the Department of Pharmacology of the Faculty of Medicine at the Erciyes University (Turkey). After studying Pharmacy at Ankara Hacettepe University, she graduated from Erciyes University in 2002 with an MS degree. She received her doctorate degree from the same institution, Faculty of Medicine, Department of Pharmacology in 2007. During her doctorate, she had the opportunity to work in the Department of Pharmacology of the Polish Academy of Sciences in Krakow, Poland. She also teaches at the Genkok Genome and Stem Cell Center, Department of Molecular Biology and Genetics, Erciyes University. Currently, her interests focus on stem cells, cancer treatment and prevention of chemotherapy-related side effects.
AMP(K)lifying NRF2 signaling
Shara Natalia Sosa Cabrera 1,2, Eleni Petsouki 1, and Elke H. Heiss 1
1 Department of Pharmaceutical Sciences, University of Vienna, 1090 Vienna, Austria; eleni.petsouki@univie.ac.at (E.P), elke.heiss@univie.ac.at (E.H.H.)
2 Vienna Doctoral School of Pharmaceutical, Nutritional and Sport Sciences, University of Vienna, 1090 Vienna, Austria
Email: shara.natalia.sosa.cabrera@univie.ac.at
Life is continuously exposing our cells to stressful insults. To adapt /restore homeostasis and prevent damage, several mechanisms evolved that fight and counteract these stressors and lend cells resilience. One player in the context of pending oxidative and xenobiotic harm is the transcription factor nuclear factor erythroid 2- related factor 2 (Nrf2) (Osama et al., 2020). In the context of metabolic/energy stress, AMP-activated protein (AMPK) enters the stage as main sensor of cellular energy status in all eukaryotic cells (Garcia and Shaw, 2017)
and central regulator of energy homeostasis. BACH1 is also a transcription factor that competes with Nrf2 to bind to the sMAF and thereby negatively regulates the transcription of antioxidant genes. Furthermore, BACH1 also regulates some genes related to glycolysis, motility (e.g mmp1) and positively correlates with cancer malignancy (Padilla and Lee, 2021).
We have previously shown that three AMPK-dependent phosphorylation of Nrf2 (S374, S408 and S433) trigger decreased levels of Nrf2 via enhanced bTrcP2-mediated degradation in Keap-/- deficient murine fibroblasts (Petsouki et al., 2023). This study therefore examined whether pharmacological activation of AMPK by metformin can decrease the levels of Nrf2 in KEAP -/- lung cancer cells which are resistant to chemotherapy due to Nrf2 hyperactivity.
Treatment of A549 (KEAP1 -/-, LKB1-/-) with Metformin did not alter the levels and stability of Nrf2, Nrf2 target genes or the sensitivity to anti-cancer drugs such as cisplatin, as assessed by qPCR, western blot, and cell viability experiments. However, it decreased the mRNA and protein levels of BACH1, the mRNA levels of mmp1 and cell motility (evident in a scratch assay) in a, presumably, AMPK-independent manner. The metformin treatment also increased the levels of ROS, and it blunted the glycolytic spare capacity of the cells in a BACH1 independent way.
Overall, AMPK activation by metformin cannot recapitulate the observed influence of the AMPK-dependent phosphorylation on Nrf2 protein stability, but lowers BACH1 abundance, glycolytic capacity and cellular motility in A549 lung cancer cells. Further studies are needed to elucidate the exact mechanism by which metformin mediates these effects with a special focus on causal involvement of AMPK and/or BACH1.
Garcia, D., Shaw, R.J., 2017. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 66, 789–800. https://doi.org/10.1016/j.molcel.2017.05.032
Osama, A., Zhang, J., Yao, J., Yao, X., Fang, J., 2020. Nrf2: a dark horse in Alzheimer’s disease treatment. Ageing Res. Rev. 64, 101206. https://doi.org/10.1016/j.arr.2020.101206
Padilla, J., Lee, J., 2021. A Novel Therapeutic Target, BACH1, Regulates Cancer Metabolism. Cells 10, 634. https://doi.org/10.3390/cells10030634
Petsouki, E., Ender, S., Sosa Cabrera, S.N., Heiss, E.H., 2023. AMPK-Mediated Phosphorylation of Nrf2 at S374/S408/S433 Favors Its βTrCP2-Mediated Degradation in KEAP1-Deficient Cells. Antioxidants 12, 1586. https://doi.org/10.3390/antiox12081586
Media composition and oxygen tension modulate NRF2 activation in HEPG2 cell line
Rutt Taba, Marie Põlluaed, Marju Puurand, Karin Tein, Tuuli Kaambre and Anton Terasmaa
Laboratory of Chemical Biology, National Institute of Chemical Physics and Biophysics, Akadeemia tee 23, 12618 Tallinn, Estonia
Email: anton.terasmaa@kbfi.ee
Cell models play a central role in preclinical research aimed to reveal the molecular mechanism of disease and drug discovery. Alterations in cell antioxidant response are at the root of many diseases. Therefore, in vitro model of disease must also faithfully reproduce these disturbances. The activity of antioxidant response is regulated by the outside environment of the cells, including levels of nutrients and oxygen tension. At the same time, the composition of commonly used cell media deviates significantly from the composition of interstitial fluid in our body. The aim of this study was to evaluate the effect of substitution of classic cell media (DMEM) with cell media in which the composition of nutrients matches human plasma (Plasmax) on cell viability and and activation of NRF2. For reasons of convenience, traditional cell culture work is done at ambient oxygen pressure (21 kPa) while partial pressure of oxygen is just 4-5 kPa in most tissues. Results show that stress tolerance and activation of NRF2 in HEPG2 cell line grown in physiological media depends on the growth conditions.
These results illustrate the importance of finding proper conditions on cell growth and the effect it has on cell viability, antioxidant response and mitochondrial respiration.
Anton Terasmaa studied chemistry at the University of Tartu, Tartu, Estonia (1997) and neuroscience at Karolinska Institutet, Stockholm, Sweden (PhD 2004). Current position is senior research fellow at National Institute of Biophysics and Chemical Physics, Talllinn, Estonia. Previous research interest was characterization of animal models of Wolfram syndrome and drug repurposing for this disease. Current research interest focuses on in-vitro modeling of neurodegeneration with brain iron accumulation (NBIA). Our aim is to develop a genetically modified cell model of a NBIA that can serve as a tool that will accelerate drug discovery for these disorders.
Investigating the Crosstalk Between Intracellular Free Zn2+ and Nrf2 Signaling in High Sucrose Diet-Induced Metabolic Syndrome Heart
Leila Aryan1, Suatnur Şık1, Firat Akat1,2, Erkan Tuncay1,3
1 Department of Biophysics, Ankara University, Faculty of Medicine, Ankara, Turkey 2 Department of Physiology, Ankara University, Faculty of Medicine, Ankara, Turkey 3 Department of Neuroscience, Ankara University, Faculty of Medicine, Ankara, Turkey
Email: etuncay@medicine.ankara.edu.tr
Background&Aim: Metabolic syndrome (MetS) represents a series of cardiovascular risk factors, in particular, dyslipidemia, hypertension, and insulin resistance (IR). The underlying mechanism of MetS induced cardiac dysfunction still needs to be revealed. MetS-induced electrical and mechanical depression, increased reactive oxygen species (ROS), and increased intracellular free zinc [Zn 2+]i was widely reported. We aimed to examine the possible role of MetS-induced [Zn2+]i and K-acetylation alterations on Nrf2 signaling.
Materials&Methods: 2-month-old, male Balb/c mice were randomly divided into two groups, Control (C), and Metabolic Syndrome (MetS). High-sucrose solution (32% sucrose) was given as drinking-water for 15weeks to animals in the MetS group. Mice myocytes were isolated for further analysis. At the same time, H9c2 cells were incubated with palmitic acid (50µM for 24h) to establish the IR-H9c2 model. Then cells were incubated with SIRT1 activator (SRT1720; 2µM for 24h) or inhibitor (EX5720; 10µM for 24h) to alter the SIRT1-dependent K-acetylation. Subcellular free Zn2+ distributions were determined by using Zn2+- sensitive fluorescent FRET-sensor and protein levels by Western-blotting. Moreover, H9c2 cells were incubated with zinc ionophore, Zn2+‐pyrithione (ZnPT, 3 and 30 nM; 24h) to increase the [Zn2+]i. The mitochondrial membrane potential (MMP), [Zn2+]i, and ROS production levels were determined by using fluorescence techniques.
Results: We observed depolarized MMP, increased ROS, [Zn2+]i, and K-acetylation both in MetS mice and IR-H9c2. SIRT1, Nrf2, and Keap1 protein levels were decreased significantly in MetS and IR-H9c2. Incubation of the control H9c2 cells with SRT1720 elevated the K-Acetylation, ROS, [Zn2+]i, and [Zn2+]Mit and decreased the Nrf2 and Keap1 protein levels. On the contrary, incubation of the IR-H9c2 cells with EX5720 normalized all parameters altered by IR. ZnPT incubation reduced the protein level of Nrf2 and Keap1.
Conclusion: Our results suggest that MetS-induced increased K-Acetylation through SIRT1 inhibition increases the [Zn2+]i and it may inhibit the Nrf2/Keap1 signaling by decreasing the protein levels and contributing to ROS production in cardiomyocytes (Supported by TUBITAK-SBAG-222S502).

Erkan Tuncay is an associate professor in Biophysics. He completed his MSc and PhD in the Department of Biophysics at Ankara University. His research is mainly focused on cardiac electrophysiology, particularly diabetic cardiomyopathy and heart failure. He has an interdisciplinary background with outstanding academic experience in various fields including physics, physiology, biology, imaging, medical device design, and biophysics. He had been at Imperial College London and New York University for his PostDoc studies. As a PI and co-investigator in national and international projects, he laid the groundwork for the proposed position by studying the effects of NAD+-dependent deacetylases, and intracellular ions on glucose homeostasis, protein trafficking, electrical and mechanical activity of the contractile cells in aging, insulin-resistant and diabetic animal models. With his active engagement in numerous national and international collaboration projects, he has earned distinguished international recognition for research excellence. He has published over 50 articles and holds 2 patents.
Discovery of Novel and Potent Inhibitors Against Nrf2–Keap1 Complex via Structure-Based Drug Design Approach and in silico Screening Methods
Kemal Yelekci
Kadir Has University, Computational Biology Research Group
Email: yelekci@khas.edu.tr
Kelch-like ECH protein 1(Keap1)-Nuclear factor erythroid 2-related factor 2 (Nrf2) complex has become an attractive drug target because of its involvement in neurodegenerative diseases such as Parkinson’s , Alzheimer’s disease, multiple sclerosis, and amyotrophic lateral sclerosis. Moreover, the Nrf2 pathway represents a natural cell defense mechanism by controlling the physiological and pathological symptoms generated from the ROS and electrophiles. In this study, the x-ray crystal structure of the human Keap1 Kelch domain (PDB: 5fzn) was downloaded, water molecules were removed all hydrogens were added. Biovia DS 4.5 Studio protocol was used to optimize the structure by selecting the “Clean Geometry” toolkit with a fast, Deriding-like force- field. Further, energy minimization of the protein was carried out utilizing the “Prepare Macromolecule” protocol of DS with the assignment of CHARMM force field based on the protonation state of the titratable residues at physiological pH 7.4. Validation studies of the final macromolecule were carried out with experimentally known inhibitors. ZINC15 and Chembl small molecule libraries were used for in silico screening and docking work. Those molecules having higher binding energies above the threshold values against the Keap1 target were selected. Their poses and interactions with the amino acid residues in the binding cavity were visualized. The molecular dynamic behaviors of these final compounds were investigated by carrying out additional MD simulation work. As a result of this study, some compounds were selected as the most promising lead for the future development of novel drug targets against the Keap1-Nrf2 target.

Kemal Yelekci graduated from Middle East Technical University, Chemistry Department. He was granted the Fulbright scholarship to pursue his Ph.D. degree in Ohio University in synthetic organic chemistry. He worked at Northwestern University (Chicago, USA) as a Post Doc. in medicinal chemistry and drug design and synthesis. Research and development involving studies of drug design and drug action, receptor ligand interaction and transporter protein mechanisms. In silico screening, calculation of free energy profile, elucidation of the flexibility and dynamical behavior of the protein-substrate complex are some of the major goals. Molecular docking methods to investigate the structure, kinetics and thermodynamics of biological molecules, especially enzyme-ligand complexes.
Development of New Inhibitors for KEAP1-NRF2 Protein-Protein Interaction with Molecular Modeling Applications
Dilruba Büşra Çakir1, Gizem Tatar Yilmaz2,*
1 Department of Bioinformatics, Institute of Health Sciences, Karadeniz Technical University, Trabzon, Turkey 2 Department of Bioinformatics, Institute of Health Sciences, Karadeniz Technical University, Trabzon, Turkey *Corresponding author
Email: gizemtatar@gmail.com
Inflammation and oxidative stress play an important role in the pathogenesis of many diseases. Nrf2 (nuclear NFE2-associated factor-2), the NFE2L2 gene in humans in the regulation of the cellular antioxidant response, encoded by is a key transcription factor. Also, Nrf2 is an essential leucine zipper (bZIP) protein that can regulate the expression of the antioxidant protein that protects against oxidative stress caused by inflammation or damage. The Keap1-Nrf2-ARE pathway is the major protection mechanism that neutralizes oxidative stress. This pathway has a protective effect on many diseases such as cancer, cardiovascular diseases, diabetes, and autoimmune diseases. Consequently, the protein-protein interaction between Keap1 and Nrf2 has emerged as a significant target for drug development. The primary objective of this study is to investigate the impact of protein-protein interactions on the molecular functions of the KEAP1-NRF2 pathway. Additionally, the study aims to identify novel inhibitors for the Keap1-Nrf2 protein-protein interaction and enhance the efficiency of Nrf2-ARE signaling pathway activation through the utilization of molecular modeling techniques. Our results reveal significant interactions that play a crucial role in ligand binding to KEAP1 and emphasized the importance of ligand-induced conformational changes in KEAP1 residue Asn414, Arg483, Asn382, Ser508, and His575, which facilitate access to a deep hole located at the bottom of the binding site. The analysis conducted in this study will contribute significantly to the development of novel inhibitors targeting KEAP1-NRF2 interactions.
Dilruba Büşra ÇAKIR graduated with a bachelor’s degree in Genetics and Bioengineering in 2018. Currently, she is a master’s student at Karadeniz Technical University, Institute of Health Sciences, Department of Bioinformatics in Trabzon. Her research areas are based on bioinformatics, computer-aided drug design (CADD), and Next Generation Sequencing (NGS). A significant part of her studies has been focusing on the determination of protein structure & dynamics and understanding the molecular interaction between protein and protein or ligand through computational molecular modeling (in silico) methods.
- 17:20 – 19:20 Joint WG Meetings Chairs: All WG leaders
- 19:20 – 19:30 Concluding Remarks and Outlook: Where to go from here? Antonio Cuadrado, Spain
- 17:30 – 19:00 Guided City Tour of Old Town of Graz (start: meeting venue, end: hotel Mercure)
- 20:00 – 23:00 Conference Dinner | Grand Hôtel Wiesler