NRF2 in COST Action 20121
Our quarterly newsletter attempts to provide our latest news and also aims at becoming a forum for analysis of relevant topics on the field of NRF2, and provide comments to some of the most relevant articles published during the quarter. Previous newsletters can be accessed at:
https://benbedphar.org/our-first-newsletter/
https://benbedphar.org/issue-2-abril-2022/
https://benbedphar.org/issue-3-july-2022/
https://benbedphar.org/issue-4-october-2022/
We have started the second grant period. A lot of exciting activities have been planned and we can mark our agendas for the following events:
- Hybrid meeting of the Management Committee (April 19 th ) and Scientific meeting (April 20-21) to be held at Zagreb (Croatia) organized by Profs. Ana Cipak and Lidija Milckovic.
- Training school on “NRF2 in noncommunicable diseases: from bench to bedside” (June 26-30) in Smolenize Castle, close to Bratislava (Slovakia) organized by Prof. Iveta Bernatova and her team.
- Scientific meeting on October 12-13, in Gratz (Austria) organized by Profs. Brigitte
Winklhofer-Roob and Christina Morgenstern. - Regarding the meeting of the SFRR-E that will be held in Vienna (June 6–9) some of us nominated Prof. Masayuki Yamamoto for the SFRR-E Annual Award Lecture and we are very pleased to see that he received this award. We encourage BenBedPhar members to register for this excellent meeting and have the opportunity to listen to the excellent panel of speakers, including Prof. Yamamoto.
I big change of the Action will be the transfer of the Grant Holder to the “Victor Babes” National Institute of Pathology, Bucharest, Romania. We hope that this change will help with the administration of expenses and in economic savings. As soon as the new agreements are signed with COST we will be able to open again the applications for Short Term Scientific Missions (STSMs).
Regarding other activities, several special issues are open for submission of review or experimental studies connected with BenBedPhar:
https://benbedphar.org/special-issue-oxidative-stress-and-nrf2-in-health-and-disease/
https://benbedphar.org/special-issue-crosstalk-between-oxidative-stress-and-
inflammation-in-cardiovascular-diseases-cancer-and-neurodegeneration/
https://benbedphar.org/special-issue-role-of-the-transcription-factor-nrf2-in-
neurodegenerative-disorders/
Antonio Cuadrado
Chair of COST Action 20121, BenBedPhar
Autonomous University of Madrid
Opportunities of collaboration among BenBedPhar members
Our COST Action provides an excellent base to apply for joint grants. As indicated in the December newsletter, several grants have been awarded to collaborative teams and new opportunities are around the corner. In this section we call your attention for i) a successful joint application to the prestigious foundation La Caixa, ii) a new opportunity based on the use of cohorts provided by WG3, and iii) a list of next calls that might be of interest for BenBedPhar participants.
A case of success: A new treatment to prevent age-related macular degeneration (AMD)
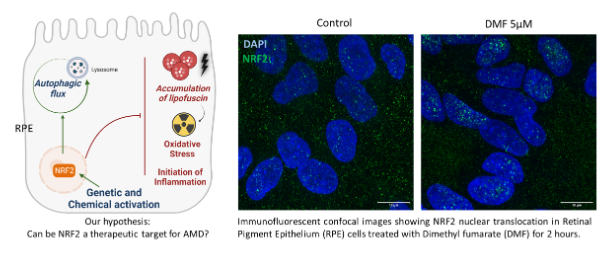
A project led by Prof. Miguel Seabra, from Nova Medical School, Universidade NOVA de Lisboa (NMS|UNL), in collaboration with Prof. Sandra Tenreiro (NMS|UNL), and having as partner Prof. Antonio Cuadrado, from Universidad Autónoma de Madrid, was funded with ~1 million euros from “la Caixa” foundation to investigate new NRF2-based treatments to prevent or modify progression of age-related macular degeneration (AMD). AMD is a degenerative disease of the macula that causes a progressive impairment of the central vision and is the leading cause of irreversible blindness in developed countries and is currently incurable. The disease is caused by the death of a specific type of cell named retinal pigment epithelium, in the area of the retina responsible for high-resolution vision, the macula. The researchers have found strong new evidence that the NRF2 plays a key role preventing these cells from dying in a model of AMD. Accordingly, this “la Caixa” financed project will explore the protective role that NRF2 may play in AMD before it is irreversible and causes permanent damage. Laboratory-based studies will aim at selecting the best molecule that activates NRF2 and protects the retina. These studies will be complemented by a clinical pilot observational study in patients, who are prescribed an NRF2-activating drug already in clinical use. The results of this study could pave the way towards the development of a much-sought after AMD preventive treatment, as well as other chronic age-related diseases.
Prof. Sandra Tenreiro
Universidade NOVA de Lisboa
Portugal
Call for expressions of interest: unique opportunity for members of BenBedPhar
Prof. Brigitte Winklhofer-Roob from the University of Graz is sharing samples and data of a cohort derived from an FP7 Framework Program entitled BIOCLAIMS to interested researchers. The cohort is already exceptionally well characterized and different types of samples are available for additional analysis, along with a comprehensive data collection. If you are interested in receiving samples, please send a message to brigitte.winklhoferroob@uni-graz.at for further information.
Cohort description: The BIOCLAIMS cohort consists of a total of 1310 study subjects, 606 males (M) and 704 females (F), covering the entire age range of 18-85 years (mean 51.3±16.1 years), and recruited prospectively according to pre-defined inclusion/ exclusion criteria. Blood was collected after an overnight fast as were spot urine and 24h urine samples, buccal mucosal cells, hair and nails. Standardized pre-analytical sample work-up was performed to ensure best possible sample quality (over 100 aliquots, including blood cells, RNA, DNA) (BIOCLAIMS sample collection). All study subjects underwent comprehensive dietary (240-item quantitative food-frequency questionnaire, prospective weighed 5-day food records), anthropometric (body mass index; skinfold thicknesses; subcutaneous adipose tissue distribution at 15 body sites using lipometer®; bioelectrical impedance measurements), clinical (blood pressure; intima-media thickness of the carotid artery; flow-mediated dilatation of A. brachialis), and biochemical characterization (clinical chemistry; vitamins and trace elements; oxidative stress including transcription factor activation; adipose tissue-derived and plasma metabolome biomarkers, including amino acids, acyl-carnitines and fatty acids), resulting in over 500 variables, including pro-inflammatory genetic polymorphisms, for each subject to date. Subsets of the volunteers further participated in the (i) Menstrual Cycle Study, including 4 investigations of 28 women at early and late follicular and early and late luteal phase; (ii) Seasonal Variability Study, including 12 investigations of 52 study subjects (M=F) at monthly intervals; and (iii) Day-to-Day Variability Study, including 5 investigations Monday-Friday of 12 study subjects (M+F). Samples of distinct subsets of the cohort have been further investigated in the BIOCLAIMS Integration (BIG) Study to validate new biomarkers in subjects with mildly impaired renal, vascular and metabolic health status in comparison to a group of so-called “super healthy” subjects, using sophisticated algorithms. Additional subset analysis focused on, for instance, high versus low intake of bioactive food compounds and different levels of adherence to a Mediterranean or Cafeteria-type diet. Given its comprehensive characterization, along with the availability of stored samples for future analysis, the BIOCLAIMS cohort constitutes a strong asset for discovery and validation of biomarkers of human health.
Next coming Grant calls
- BSI Career Enhancing Grants – Immunology; Deadline for submission is Monday, March 27th, 2023. https://www.immunology.org/membership/grants-prizes/bsi-career-enhancing-grants
- Drug development RFP – Alzheimer’s disease; Upcoming deadlines for submission are February 3rd, 2023 and May 19th, 2023. https://www.alzdiscovery.org/research-and-grants/funding-opportunities/drug-development-rfp
- CurePSP – Neurodegenerative diseases; Deadline for submission is March 17th, 2023. https://www.psp.org/iwanttolearn/grants/#page-section-research-grants
- Pfizer Research Grant RFP Junior Investigator Global ATTR Amyloidosis Research ASPIRE – Deadline for submission is Monday, March 21st, 2023. https://cdn.pfizer.com/pfizercom/2023-01/2023%20RD%20G%20-%20Global%20ATTR%20Amyloidosis%20Research.pdf?BVNX6t3YH3b0jUjJRYDpPvX_noZqu3ld Other funding opportunities: https://www.pfizer.com/about/programs-policies/grants/competitive-grants
- HORIZON-HLTH-2024-TOOL-05-06-two-stage – Opening on March 30th, 2023. Deadline for submission September 19th, 2023. https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/opportunities/topic-details/horizon-hlth-2024-tool-05-06-two-stage
- Human Frontier Science Program – Deadline for submission is Monday, March 21st, 2023. https://www.hfsp.org/funding/hfsp-funding/research-grants
Comments from the Working groups
Can vitamin D supplementation help overcome winter blues by changing NRF2 level?
Vitamin D is made primarily in our skin following sunlight exposure; therefore, its level should be above 52 nmol/L (measured as serum total 25(OH)D [Ferrari D et al., Biochem. Med. (Zagreb) 2017]).

Remarkably, vitamin D deficiency is very common around the world, especially during the winter months, when it is advised to be supplemented [Holick M.F., N Engl. J. Med. 2007]. Vitamin D is necessary for healthy bones and muscles, but is also known for its non-canonical effects associated with age-related diseases or oxidative stress. Although vitamin D does not have direct free radical scavenger activity like vitamin C or E, it upregulates essential proteins for a cellular response such as NRF2 and antioxidant enzymes, e.g., glutathione peroxidase and reductase. Furthermore, vitamin D points to antagonise senescent cells, as it activates antiapoptotic, antiproliferative and prodifferentiative mechanisms [Sosa-Diaz E. et al., Free Radical Biol. Med 2022].
Recent studies highlight the crosstalk between vitamin D and NRF2. For example, vitamin D directly regulates NRF2 expression through the vitamin D receptor (VDR) and the VDRE region that is present in the NRF2 gene [L. Chen et al., Ageing Cell 2019; Li et al., Mol. Med. 2022]. Vitamin D may also bind to the Keap1 protein; thus, it interferes with Keap1 binding to NRF2, leading to activation of antioxidant mechanisms in cells [Uthaiah et al., Antioxidants 2022]. In addition to direct activation of NRF2, vitamin D can indirectly activate NRF2. Scuto et al. showed that vitamin D influences p62, a marker of autophagy. After activation, p62 dissociates Keap1 from NRF2, triggering its activation [Mech. Ageing Dev. 2021]. Furthermore, p62 facilitates the heterodimerization of VDR with RXR, supporting its activation and, indirectly, the activation of NRF2 [Duran et al., Cancer Cell 2016]. Therefore, vitamin D can modulate NRF2 transcriptional activity. Studies in patients and mice showed that vitamin D supplementation reduces clinical and metabolic symptoms in patients with end-stage renal disease [Sharif E., Cell Mol Biol (Noisy-le-grand) 2022] and reverses premature aging in 1,25(OH)2D3-deficient [1α(OH)ase−/–] mice [Chen et al., Ageing Cell 2019].
Aleksandra Piechota-Polanczyk
WG1 and WG3 member
Jagiellonian University, Poland
Targeting KEAP1 with PROTACs
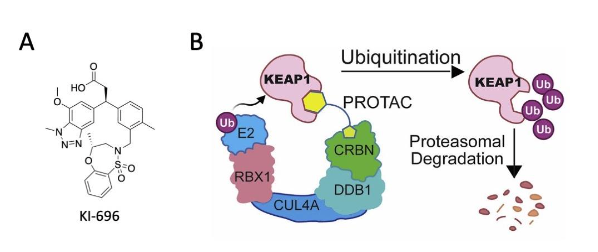
Targeted protein degradation has the potential to experimentally and therapeutically modulate proteins that are difficult to target with conventional small molecules. One approach of achieving targeted protein degradation is through proteolysis-targeting chimeras (PROTACs). PROTACs are heterobifunctional small molecules that comprise of two components connected by a linker; one of the PROTAC components binds the protein of interest, whereas the second component binds and recruits E3 ubiquitin ligase (Sakamoto et al. 2001). Thus, this strategy allows the simultaneous binding of the protein of interest and the ligase, resulting in the ubiquitylation and subsequent proteasomal degradation of the protein of interest (Békés et al. 2022). Recently two independent groups (Du et al. 2022 and Chen et al. 2022) have employed this strategy to design a heterobifunctional degraders of Kelch-like ECH associated protein 1 (KEAP1), the principal negative regulator of NRF2. In both cases, these KEAP1-targeting PROTACs were based on the small molecule KI696 (Figure 1A), which was originally designed to bind with high affinity to the Kelch domain of KEAP1 and potently inhibit the protein-protein interactions between KEAP1 and NRF2, leading to NRF2 activation (Davies et al. 2016). To create these PROTACs, KI696 was linked to a ligand that binds the Cullin4-Rbx1 ligase-cereblon (CRBN) complex, resulting in the CRBN-dependent ubiquitination and subsequent degradation of KEAP1 (Figure 1B). A very important feature of this strategy, which is in contrast with classical small molecule inhibitors, is that, following the degradation of the protein of interest, the PROTAC is recycled to target another molecule of the protein of interest, making this mechanism of action catalytic and thus highly efficient.
Additionally, small-molecule or peptide-based PROTACs have been developed to generate KEAP1-recruiting degraders that lead to the degradation of proteins, such as the bromodomain and extra-terminal (BET) family members BRD3 and BRD4, focal adhesion kinase and Tau (Lu et al. 2018; Wei et al. 2021; Du et al. 2022), demonstrating the possibility that the E3 ligase function of KEAP1 can be harnessed for the degradation of proteins that are difficult to target and are not physiological substrates for the Cullin3-Rbx1 ligase-KEAP1 complex.
References
Békés et al. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21:181-200.
Chen et al. Design and characterization of a heterobifunctional degrader of KEAP1. Redox Biol. 2022;59:102552.
Davies et al. Monoacidic Inhibitors of the Kelch-like ECH-Associated Protein 1: Nuclear Factor Erythroid 2-Related Factor 2 (KEAP1:NRF2) Protein-Protein Interaction with High Cell Potency Identified by Fragment-Based Discovery. J Med Chem. 2016;59(8):3991-4006.
Du et al. Exploring the target scope of KEAP1 E3 ligase-based PROTACs. Cell Chem Biol. 2022;29:1470-81.
Lu et al. Discovery of a Keap1-dependent peptide PROTAC to knockdown Tau by ubiquitination-proteasome degradation pathway. Eur J Med Chem. 2018;146:251-9.
Sakamoto et al. PROTACs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci U S A. 2001;98:8554-9.
Wei et al. Harnessing the E3 Ligase KEAP1 for targeted protein degradation. J Am Chem Soc. 2021;143:15073-83.
Albena T. Dinkova-Kostova
WG2 leader
School of Medicine, University of Dundee, United Kingdom
Co-exposure to urban particulate matter and aircraft noise adversely impacts the cerebro-pulmonary-cardiovascular axis in mice
Worldwide, up to 8.8 million excess deaths/year have been attributed to air pollution, mainly due to the exposure to fine particulate matter (PM). Traffic-related noise is an additional contributor to global mortality and morbidity. Both health risk factors substantially contribute to cardiovascular, metabolic and neuropsychiatric sequelae. Studies on the combined exposure are rare and urgently needed because of frequent co-occurrence of both risk factors in urban and industrial settings. To study the synergistic effects of PM and noise, we used an exposure system equipped with aerosol generator and loud-speakers, where C57BL/6 mice were acutely exposed for 3d to either ambient PM (NIST particles) and/or noise (aircraft landing and take-off events). The combination of both stressors caused endothelial dysfunction, increased blood pressure, oxidative stress and inflammation. An additive impairment of endothelial function was observed in isolated aortic rings and even more pronounced in cerebral and retinal arterioles. The increase in oxidative stress and inflammation markers together with RNA sequencing data indicate that noise particularly affects the brain and PM the lungs. Heme oxygenase-1 upregulation, probably via NRF2 activation, represents a stress response to PM and noise, which may be responsible for the rather moderate additive effects of the exposures. NRF2 activation and heme oxygenase-1 induction were previously shown to prevent noise-induced cardiovascular damage. The combination of both stressors has additive adverse effects on the cardiovascular system that are based on PM-induced systemic inflammation and noise-triggered stress hormone signalling. We demonstrate an additive upregulation of ACE-2 in the lung, suggesting that there may be an increased vulnerability to COVID-19 infection. The data warrant further mechanistic studies to characterize the propagation of primary target tissue damage (lung, brain) to remote organs such as aorta and heart by combined noise and PM exposure.
https://pubmed.ncbi.nlm.nih.gov/36566737/
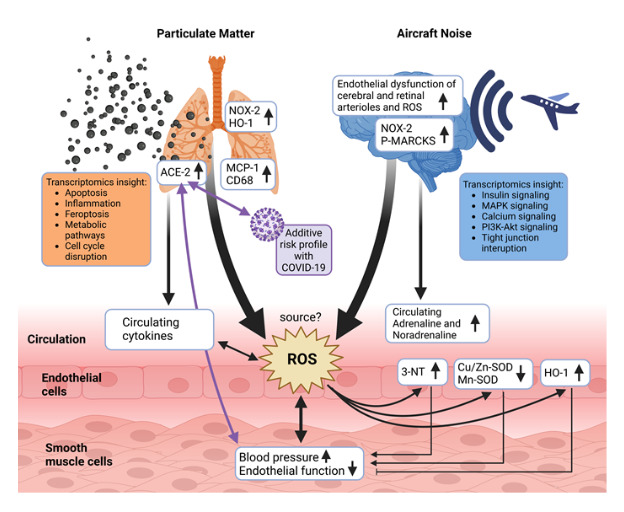
Summarizing scheme. Proposed pathomechanisms triggered by noise, PM or combined exposure. While PM confers most of its detrimental effects via damage of the lung (inflammation and oxidative stress), noise initiates systemic adverse health effects primarily by causing neuronal activation, cerebral oxidative stress and stress hormone release. The primary target organ damage by PM and noise converges at the cardiovascular level showing additive exacerbation of some functional (endothelial dysfunction) and biochemical markers (oxidative stress by 3-NT, SODs, HO-1). The source of reactive oxygen species (ROS) in the vasculature was not yet entirely identified but may be a mixture of different NADPH oxidase enzymes and mitochondrial ROS sources. The elevated ACE-2 expression in the lungs of PM and noise co-exposed mice may not only increase the risk and severity of COVID-19 infections but also have general effects on cardiovascular health. Scheme was created using Biorender program. From Kuntic et al. Redox Biology 2022 (DOI: 10.1016/j.redox.2022.102580).
Marin Kuntic, Ivana Kuntic, Roopesh Krishnankutty et al., Alex von Kriegsheim, Andreas Daiber, Thomas Münzel
WG3
Mainz, Germany
Update on the effects of omaveloxolone, NRF2 activator, in patients with Friedreich ataxia (FRDA)
MOXIe was a two-part study evaluating the safety and efficacy of omaveloxolone in patients with Friedreich ataxia (FRDA), a rare and progressive neurological disease with no proven therapy. Omaveloxolone, is a nuclear factor erythroid 2-related factor 2 (NRF2) activator, and is currently under review by the Food and Drug Administration (FDA) and has the potential to be the first approved treatment for FRDA. Reata Pharmaceuticals, a clinical-stage company, recently has published the uptade of the results obtained in this clinical trial. The open-label extension enrolled 149 patients (87% of those enrolled in part 1 or part 2) and remains ongoing.
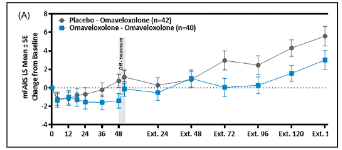
The results presented in the publication of the delayed-start analyses indicate a persistent benefit of omaveloxolone treatment on disease course modified Friedreich’s Ataxia Rating Scale (mFARS) scores relative to placebo.
The previous Study 1402 part 2 analysis included data through part 2 week 48, now, the delayed-start analysis includes that part 2 data plus the data from all visits through extension week 144 as of the database lock.
MOXIe part 2, a randomized double-blind placebo-controlled trial, showed that double-blind period experienced a sustained benefit that could not be recovered by individuals initially randomized to placebo who began omaveloxolone in the extension study, implying a benefit of starting omaveloxolone treatment earlier. Patients previously randomized to omaveloxolone in the controlled, double-blind period continue to show disease progression, as assessed with mFARS, over more than 2.5 years of treatment in the extension; mean mFARS values for these individuals were maintained from extension baseline through extension week 144. The delayed-start analysis suggests that the improvement in neurological function persists to some degree with ongoing therapy, suggesting that earlier treatment with omaveloxolone might provide greater benefit than delayed therapy.
In summary, the actual results suggest that the NRF2 activator omaveloxolone is generally safe and well tolerated and presents a persistent beneficial effect on the disease course in FRDA, indicating that omaveloxolone may be an efficacious therapy in the treatment of FRDA.
Santiago Cuevas
WG4 leader
BioMedical Research Institute of Murcia (IMIB), Murcia, Spain
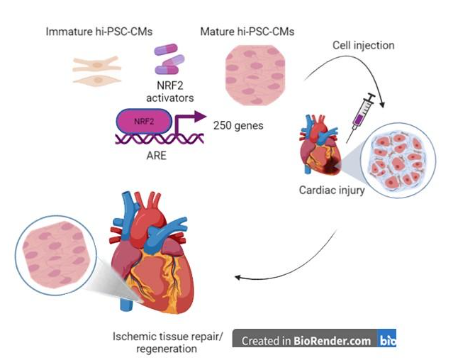
NRF2 activation as new tool to repair damaged tissues in cardiovascular ischemic diseases
In the last years, there has been a growing interest about the potential role of stem/progenitor cells in the cardiovascular regeneration after myocardial infarction and in chronic ischemic heart. The prevailing cause of heart failure is the death of heart muscle tissue. Cardiomyocyte loss and inflammation are detrimental as they lead to pathological remodelling, reduced myocardial function and inevitable progression to heart failure. Exogenous transfer of a variety of stem/progenitor cells resulted safe, orchestrate functional improvement and ischemic tissue repair/regeneration after cardiac injury [1,2]. However, there are still several critical factors that determine the success of the regenerative capacity of transplanted cells and make their use difficult (e.g. very little or no replacement of lost cells, immature phenotype, teratoma developing).
A recent study published in the Stem Cell Research & Therapy is perhaps the first to show that NRF2 activation may confer a mature phenotype in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) [3]. Zhang and co-workers showed that after activation of NRF2 by knocking down KEAP1 expression, hiPSC-CMs displayed key cardiomyocyte phenotypic characteristics more closely resemble mature cardiomyocytes in the morphology and cardiac markers. In hiPSC-CMs, NRF2 activation also promoted a change in the energy metabolism from glycolysis to oxidative phosphorylation of fatty acids thus directly regulating the expression and function of mitochondrial respiratory complexes.
This study supports the importance of investigate on pharmacological NRF2 activators that target energy metabolism, oxidative stress and inflammation thus providing an opportunity to greatly improve understanding of how to augment the number and function of progenitor cells to repair damaged tissues (Figure 1). However, far more studies are warranted to fully understand the role of the NRF2 activation in other pluripotent stem cell lines and to delineate more comprehensive mechanistic insights.
References
- Manginas, A.; Goussetis, E.; Koutelou, M.; Karatasakis, G.; Peristeri, I.; Theodorakos, A.; Leontiadis, E.; Plessas, N.; Theodosaki, M.; Graphakos, S.; et al. Pilot study to evaluate the safety and feasibility of intracoronary CD133+ and CD133− CD34+ cell therapy in patients with nonviable anterior myocardial infarction. Catheter. Cardiovasc. Interv. 2007, 69, 773–781
- Fernández-Avilés F, Sanz-Ruiz R, Bogaert J, Casado Plasencia A, Gilaberte I, Belmans A, Fernández-Santos ME, Charron D, Mulet M, Yotti R, Palacios I, Luque M, Sádaba R, San Román JA, Larman M, Sánchez PL, Sanchís J, Jiménez MF, Claus P, Al-Daccak R, Lombardo E, Abad JL, DelaRosa O, Corcóstegui L, Bermejo J, Janssens S. Safety and Efficacy of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients With ST-Segment Elevation Myocardial Infarction and Left Ventricular Dysfunction. Circ Res. 2018 Aug 17;123(5):579-589. doi: 10.1161/CIRCRESAHA.118.312823. PMID: 29921651.
- Zhang X, Ye L, Xu H, Zhou Q, Tan B, Yi Q, Yan L, Xie M, Zhang Y, Tian J, Zhu J. NRF2 is required for structural and metabolic maturation of human induced pluripotent stem cell-derived ardiomyocytes. Stem Cell Res Ther. 2021 Mar 24;12(1):208. doi: 10.1186/s13287-021-02264-2. PMID: 33762018; PMCID: PMC7992990.
Brigitta Buttari
WG5 leader
Istituto Superiore di Sanità, Italy
Hot from Pubmed
Fresh Medium or L-Cystine as an Effective Nrf2 Inducer for Cytoprotection in Cell Culture
The Nrf2 gene encodes a transcription factor best known for regulating the expression of antioxidant and detoxification genes. A long list of small molecules has been reported to induce Nrf2 protein via Keap1 oxidation or alkylation. Many of these Nrf2 inducers exhibit off-target or toxic effects due to their nature as electrophiles. In searching for non-toxic Nrf2 inducers, the authors found that a culture medium change to fresh DMEM is capable of inducing Nrf2 protein in HeLa, HEK293, AC16 and MCF7 cells. Testing the components of DMEM led to the discovery of L-Cystine as an effective Nrf2 inducer. L-Cystine induces a dose-dependent increase of Nrf2 protein, from 0.1 to 1.6 mM. RNA-seq analyses and RT-PCR revealed an induction of multiple Nrf2 downstream genes, including NQO1, HMOX1, GCLC, GCLM, SRXN1, TXNRD1, AKR1C and OSGIN1 by 0.8 mM L-Cystine. The induction of Nrf2 protein was dependent on L-Cystine entering cells via the cystine/glutamate antiporter and the presence of Keap1. The half-life of Nrf2 protein increased from 19.4 min to 30.9 min with 0.8 mM L-Cystine treatment. L-Cystine was capable of eliciting cytoprotection by reducing ROS generation and protecting against oxidant- or doxorubicin-induced apoptosis. As an amino acid derivative, L-Cystine is considered a non-toxic Nrf2 inducer that exhibits the potential for protection against oxidative stress and tissue injury.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/36672226/
The Neuroprotective Effects and Therapeutic Potential of the Chalcone Cardamonin for Alzheimer’s Disease
Neurodegenerative diseases (ND) include a wide range of conditions that result from progressive damage to the neurons. Alzheimer’s disease (AD) is one of the most common NDs, and neuroinflammation and oxidative stress (OS) are the major factors in the development and progression of the disease. Many naturally occurring phytochemical compounds exhibit antioxidant and anti-inflammatory activities with potential neuroprotective effects. Several plant species, including Alpinia katsumadai and Alpinia conchigera, contain cardamonin (CD). CD (2′,4′-dihydroxy-6’methoxychalcone) has many therapeutic properties, including anticancer, anti-inflammatory, antioxidant, antiviral, and antibiotic activities. CD is a potent compound that can reduce OS and modulate the inflammatory processes that play a significant part in developing neurodegenerative diseases. CD has been shown to modulate a variety of signaling molecules involved in the development and progression of ND, including transcription factors (NF-kB and STAT3), cytokines (TNF-α, IL-1, and IL-6), enzymes (COX-2, MMP-9, and ALDH1), and other proteins and genes (Bcl-2, XIAP, and cyclin D1). Additionally, CD effectively modulates miRNA levels and autophagy-related CD-protective mechanisms against neurodegeneration. In summary, this review provides mechanistic insights into CD’s ability to modify multiple oxidative stress-antioxidant system pathways, Nrf2, and neuroinflammation. Additionally, it points to the possible therapeutic potential and preventive utilization of CD in neurodegenerative diseases, most specifically AD.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/36672126/
Targeting the NRF2/KEAP1 pathway in cervical and endometrial cancers
Cervical and endometrial cancers are among the most dangerous gynaecological malignancies, with high fatality and recurrence rates due to frequent diagnosis at an advanced stage and chemoresistance onset. The NRF2/KEAP1 signalling pathway plays an important role in protecting cells against oxidative damage due to increased reactive oxygen species (ROS) levels. NRF2, activated by ROS, induces the expression of antioxidant enzymes such as heme oxygenase, catalase, glutathione peroxidase and superoxide dismutase which neutralize ROS, protecting cells against oxidative stress damage. However, activation of NRF2/KEAP1 signalling in cancer cells results in chemoresistance, inactivating drug-mediated oxidative stress and protecting cancer cells from drug-induced cell death. This work shows a review of the literature on the role of the NRF2/KEAP1 pathway in cervical and endometrial cancers, with a focus on the expression of its components and downstream genes. It further examines the role of the NRF2/KEAP1 pathway in chemotherapy resistance and how this pathway can be modulated by natural and synthetic modulators.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/36641100/
The metabolite alpha-ketobutyrate extends lifespan by promoting peroxisomal function in C. elegans
Metabolism is intimately linked to aging. There is a growing number of studies showing that endogenous metabolites may delay aging and improve healthspan. Through the analysis of existing transcriptome data, the authors discover a link between activation of the transsulfuration pathway and a transcriptional program involved in peroxisome function and biogenesis in long-lived glp-1(e2141ts) mutant Caenorhabditis elegans worms. Subsequently, the authors show that supplementation with α-ketobutyrate, an intermediate of the transsulfuration pathway, extends lifespan in wild-type worms. Alpha-ketobutyrate augments the production of NAD+ via the lactate dehydrogenase LDH-1, leading to SIR-2.1/SIRT1-mediated enhanced peroxisome function and biogenesis, along with a concomitant increase in the expression of acox-1.2/ACOX1 in the peroxisomal fatty acid β-oxidation pathway. ACOX-1.2/ACOX1 promotes H2O2 formation, thereby resulting in activation of SKN-1/NRF2. This transcription factor in turn extends the lifespan of worms by driving expression of autophagic and lysosomal genes. Finally, the authors show that α-ketobutyrate also delays the cellular senescence in fibroblast cells through the SIRT1-ACOX1-H2O2-NRF2 pathway. This finding uncovers a previously unknown role for α-ketobutyrate in organismal lifespan and healthspan by coordinating the NAD+-SIRT1 signaling and peroxisomal function.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/36646719/
PM2.5 increases susceptibility to acute exacerbation of COPD via NOX4/Nrf2 redox imbalance-mediated mitophagy
The increasing abundance of fine particulate matter (PM2.5) in the environment has increased susceptibility to acute exacerbation of COPD (AECOPD). During PM2.5 exposure, excessive reactive oxygen species (ROS) production triggers a redox imbalance, which contributes to damage to organelles and disruption of homeostasis. At present, there are limited data on whether NOX4/Nrf2 redox imbalance increases susceptibility to acute exacerbation of COPD (AECOPD), and the underlying mechanism is unclear. Therefore, the current study was aimed to evaluate the role of NOX4/Nrf2 redox balance on AECOPD induced by PM2.5-CS-exposure. Here, the authors report that PM2.5 exacerbates cytotoxicity by enhancing NOX4/Nrf2 redox imbalance-mediated mitophagy. First, exposure to a low-dose of PM2.5 (200 μg/ml) significantly exacerbated oxidative stress and mitochondrial damage by increasing the ROS overproduction, enhancing the excessive NOX4/Nrf2 redox imbalance, decreasing the mitochondrial membrane potential (MMP), and enhancing the mitochondrial fragmentation that were caused by a low-dose of CSE (2.5%). Second, coexposure to PM2.5 and CSE (PM2.5-CSE) induced excessive mitophagy. Third, PM2.5 exacerbated CS-induced COPD, as shown by excessive inflammatory cell infiltration, inflammatory cytokine production and mucus hypersecretion, goblet cell hyperplasia, NOX4/Nrf2 redox imbalance, and mitophagy, these effects triggered excessive ROS production and mitochondrial damage in mice. Mechanistically, PM2.5-CS-induced excessive levels of mitophagy by triggering redox imbalance, leading to greater cytotoxicity and AECOPD; however, reestablishing the NOX4/Nrf2 redox balance via NOX4 blockade or mitochondria-specific ROS inhibitor treatment alleviated this cytotoxicity and ameliorated AECOPD. PM2.5 may exacerbate NOX4/Nrf2 redox imbalance and subsequently enhance mitophagy by increasing the ROS and mito-ROS levels, thereby increasing susceptibility to AECOPD.Access to the original article:https://pubmed.ncbi.nlm.nih.gov/36608590/
Thioredoxin-interacting protein is essential for memory T cell formation via the regulation of the redox metabolism
CD4+ memory T cells are central to long-lasting protective immunity and are involved in shaping the pathophysiology of chronic inflammation. While metabolic reprogramming is critical for the generation of memory T cells, the mechanisms controlling the redox metabolism in memory T cell formation remain unclear. The authors found that reactive oxygen species (ROS) metabolism changed dramatically in T helper-2 (Th2) cells during the contraction phase in the process of memory T cell formation. Thioredoxin-interacting protein (Txnip), a regulator of oxidoreductase, regulated apoptosis by scavenging ROS via the nuclear factor erythroid 2-related factor 2 (Nrf2)-biliverdin reductase B (Blvrb) pathway. Txnip regulated the pathology of chronic airway inflammation in the lung by controlling the generation of allergen-specific pathogenic memory Th2 cells in vivo. Thus, the Txnip-Nrf2-Blvrb axis directs ROS metabolic reprogramming in Th2 cells and is a potential therapeutic target for intractable chronic inflammatory diseases.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/36595680/
MMF induces antioxidative and anaplerotic pathways and is neuroprotective in hyperexcitability in vitro
Hyperexcitability-induced neuronal damage plays a role both in epilepsy as well as in inflammatory brain diseases such as multiple sclerosis (MS) and as such represents an important disease pathway which potentially can be targeted to mitigate neuronal damage. Dimethyl fumarate (DMF) and its pharmacologically active metabolite monomethyl fumarate (MMF) are FDA-approved therapeutics for MS, which can induce immunosuppressive and antioxidant pathways, and their neuroprotective capacity has been demonstrated in other preclinical neurological disease models before. In this study, the authors used an unbiased proteomic approach to identify potential new targets upon the treatment of MMF in glio-neuronal hippocampal cultures. MMF treatment results in induction of antioxidative (HMOX1, NQO1) and anaplerotic metabolic (GAPDH, PC) pathways, which correlated with reduction in ROS production, increased mitochondrial NADH-redox index and decreased NADH pool, independent of glutathione levels. Additionally, MMF reduced glycolytic capacity indicating individual intra-cellular metabolic programs within different cell types. Furthermore, the authors demonstrate a neuroprotective effect of MMF upon hyperexcitability in vitro (low magnesium model), where MMF prevents glio-neuronal death via reduced ROS production. These results highlight MMF as a potential new therapeutic opportunity in hyperexcitability-induced neurodegeneration.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/36521578/
Design and characterization of a heterobifunctional degrader of KEAP1
The Kelch-like ECH-associated protein 1 (KEAP1) – nuclear factor erythroid 2-related factor 2 (NRF2) signaling pathway senses reactive oxygen species and regulates cellular oxidative stress. Inhibiting KEAP1 to activate the NRF2 antioxidant response has been proposed as a promising strategy to treat chronic diseases caused by oxidative stress. Here, the authors developed a proteolysis targeting chimera (PROTAC) that depletes KEAP1 from cells through the ubiquitin-proteasome pathway. A previously developed KEAP1 inhibitor and thalidomide were incorporated in the heterobifunctional design of the PROTAC as ligands for KEAP1 and CRBN recruitment, respectively. Optimization of the chemical composition and linker length resulted in PROTAC 14 which exhibited potent KEAP1 degradation with low nanomolar DC50 in HEK293T (11 nM) and BEAS-2B (<1 nM) cell lines. Furthermore, PROTAC 14 increased the expression of NRF2 regulated antioxidant proteins and prevented cell death induced by reactive oxygen species. Together, these results established a blueprint for further development of KEAP1-targeted heterobifunctional degraders and will facilitate the study of the biological consequences of KEAP1 removal from cells. This approach represents an alternative therapeutic strategy to existing treatments for diseases caused by oxidative stress.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/36473314/
Lipoxin A4 attenuates MSU-crystal-induced NLRP3 inflammasome activation through suppressing Nrf2 thereby increasing TXNRD2
Gout is a common inflammatory disease. The activation of NLRP3 inflammasome induced by monosodium urate (MSU) crystals has a critical role in gout, and its prevention is beneficial for patients. Lipoxin A4 (LXA4) is an endogenous lipoxygenase-derived eicosanoid mediator with powerful anti-inflammatory properties. However, whether LXA4 can suppress NLRP3 inflammasome activation induced by MSU crystals remains unclear. This study aimed to investigate the protective effect of LXA4 on MSU-crystal-induced NLRP3 inflammasome activation and its underlying molecular mechanisms. The authors found that LXA4 inhibited MSU-crystal-induced NLRP3 inflammasome activation, interleukin (IL)-1β maturation, and pyroptosis. More specifically, LXA4 suppressed the assembly of the NLRP3 inflammasome, including oligomerization and speck formation of ASC, and ASC-NLRP3 interaction. Furthermore, LXA4 suppressed oxidative stress, the upstream events for NLRP3 inflammasome activation, as evidenced by the fact that LXA4 eliminated total reactive oxygen species (ROS) generation and alleviated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation and mitochondrial dysfunction. However, LXA4 also depressed the Nrf2 activation, a critical molecule in the antioxidant pathway, and then exerted an inhibitory impact on Klf9 expression and promotional impact on TXNRD2 expression, two molecules located downstream of Nrf2 in sequence. Knockdown of TXNRD2 reversed the LXA4-induced depression of ROS and NLRP3 inflammasome. Moreover, LXA4 alleviated joint inflammation and decreased the production of cleaved caspase-1 and matured IL-1β in gouty arthritis rats. Taken together, our findings demonstrate that LXA4 can attenuate MSU-crystal-induced NLRP3 inflammasome activation, probably through suppressing Nrf2 activation to increase TXNRD2 expression. The present study highlights the potential of LXA4 as an attractive new gout treatment candidate.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/36569930/
SRT1720 as an SIRT1 activator for alleviating paraquat-induced models of Parkinson’s disease
Epidemiological studies have linked herbicides and Parkinson’s disease (PD), with the strongest associations resulting from long exposure durations. Paraquat (PQ), an herbicide, induces PD-like syndromes and has widely been accepted as a PD mimetic. Currently, there is still no cure to prevent the progression of PD, and the search for effective therapeutic ways is urgent. Recently, the impairing activity of sirtuins (SIRTs), such as SIRT1, may correlate with PD etiology. However, the nonspecificity of SIRT1 agonists has made the protective mechanisms against PD unclear and hampered the therapeutic application of SIRT1. Thus, this study investigated the protective mechanism and therapeutic potential of SRT1720, a more specific agonist for SIRT1 synthesized by Sirtris, in alleviating the toxicity of PQ-induced cellular and animal models of PD. Here the authors show that SRT1720 alleviates PQ-induced toxicity in cell and animal models. Genetic silencing and pharmacological inhibition of SIRT1 attenuated SRT1720’s protection against PQ-induced toxicity. Moreover, SRT1720 not only attenuated PQ-induced increased oxidative stress and mitochondrial free radical formations but also decreased mitochondrial membrane potential. Furthermore, SRT1720 reversed PQ-induced decreased PGC-1α levels and mitochondrial biogenesis. Although PQ and SRT1720 elevated NRF2 and antioxidative enzyme levels, only PQ decreased antioxidative enzyme activity but not SRT1720. NRF2 and PGC-1α silencing attenuated SRT1720 protection against PQ-induced toxicity. SRT1720 targeted SIRT1 and activated downstream PGC-1α and NRF2 signalings to prevent PQ-induced toxicity involving oxidative stress and mitochondrial dysfunction. Thus, SRT1720 might have therapeutic potential in preventing PD.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/36379180/
Joana Miranda
University of Lisbon
Portugal


