NRF2 in COST Action 20121
Our quarterly newsletter attempts to provide not only our latest news but also aims at becoming a forum for analysis of relevant topics on the field of NRF2 and provide comments to some of the most relevant articles published during the quarter bout NRF2. You can access our first newsletter at: https://benbedphar.org/our-first-newsletter/, and our second newsletter at: https://benbedphar.org/issue-2-abril-2022/. The following paragraphs summarize the most relevant activities conducted during the last three months.
Our scientific meeting
On May 19-20 we could have our first face-to-face meeting at Madrid, with tittle “Translating NRF2 research from bed to bench”. We had 25 speakers and over 60 participants. The meeting gathered some of the most prestigious researches in the NRF2 field from EU, and presented state of the art work on NRF2 from basic mechanisms to clinical translations in several diseases. The meeting offered an excellent ground to develop friendship as a means to strengthen scientific collaborations and applications to international grants. Several Biopharmaceutical companies showcased their current pipelines of development of NRf2 activators: Keapstone Therapeutics, Kinjirushi Ltd.Co, and Stacicla SA. External researchers to the Action were also invited. You can access the programme and the abstract book at https://benbedphar.org/2nd-bedbenphar-scientific-meeting/.
Participation in other scientific conferences
BenBedPhar at “The Future of Redox Biology”, 2022.
On June 17-19, the “The Future of Redox Biology” meeting was co-organized by Prof Giuseppe Valacchi, Prof Enrique Cadenas, Prof Juan Sastre and Prof Giovanni Mann in Siena, Italy and supported by the Society for Free Radical Research Europe (SFRR-E), Society for Free Radical Research International (SFRR-I) and the Oxygen Club of California (OCC). The meeting was dedicated to the new and Early Career generation of scientists in the field of Redox Biology, sharing and transmitting to them our passion for science and, at the same time, obtaining new perspectives from their ideas. For this reason, the majority of the talks were given invited young scientists, who also participated as co-chairs of the scientific sessions. This meeting provided a unique opportunity for advancing scientific interactions between Early Career and established academics, researchers and clinicians in Redox Biology to further develop this exciting field.

Three BenBedPhar members participating in this meeting: Dr. Ana I. Rojo (Autonomous Universidad Madrid, Spain) spoke about “Redox, mitochondrial and NRF2 imbalance are present in C9ORF72-related amyotrophic lateral sclerosis”; Prof. Ron Kohen (Hebrew University of Jerusalem, Israel) spoke about “NRF2-KEAP1 Pathway: Role in Cutaneous Redox maintenance; Biological, Environmental and Chemical activators”; and Prof. Giovanni Mann (King’s College London, UK), Review Editor of “Free Radicals in Biology & Medicine”, presented opportunities of publication in our special issue of this journal “Bench To Bedside Transition For Pharmacological Regulation Of NRF2 In Non-Communicable Diseases”.
BenBedPhar at the Paris Redox Meeting 2022.
On June 22-24, the 24th annual Paris Redox Meeting entitled “Oxidative Stress Reduction, Redox Homeostasis & Antioxidants” was organized by the International Society for Antioxidants in Nutrition and Health (ISAHN) in Paris.
Four BenBedPhar members participated in this highly reputed meeting: Dr. Anna Grochot-Przeczek (Department of Medical Biotechnology, Jagiellonian University, Poland) spoke on the novel atypical functions of NRF2 and KEAP1 in endothelial cells; Prof. Gerasimos Sykiotis (Lausanne University Hospital and University of Lausanne, Switzerland) spoke on the impact of KEAP1/NRF2 signaling on thyroid physiology and disease; Dr. Santiago Cuevas (Biomedical Research Institute of Murcia, Spain) spoke on the involvement of the DJ-1/NRF2 pathway in the prevention of inflammasome activation and its possible role in diabetic nephropathy; and Dr. Arno Bourgonje (University Medical Center Groningen, Netherlands) spoke about personalized redox medicine in inflammatory bowel disease.
Of relevance to BenBedPhar’s translational focus, several presentations from the group of the President of ISANH, Prof. Harry van Goor (Department of Pathology and Medical Biology, University Medical Center, Groningen, The Netherlands), also BenBedPhar member, and his collaborators presented data on plasma free thiols as a biomarker of the organism’s redox status with a possible prognostic role in various diseases associated with oxidative stress. The BenBedPhar COST Action was presented during the talks with a kind invitation to interested Paris Redox attendees working on NRF2 to join the Action.
The main goals of the Action were explained, and the possibilities available via participation in BenBedPhar were presented, focusing on Scientific Meetings, Short-Term Scientific Missions and publications in Special Issues. Participation in Paris Redox meeting helped to increase BenBedPhar’s visibility, and as a result, several contacts have been established for future collaboration. The Organizers disseminated information about the talks of BenBedPhar members on social media (LinkedIn and Facebook). Prof. Anna Grochot-Przeczek received the award for the best conference lecture, and she received a travel grant to participate at the Paris Redox 2023 Meeting.

Webinars
In collaboration with the Sapienza University of Rome, we have been organizing a webinar series on biochemical, pharmacological and clinical aspects of NRF2. You can access the information and presentations at https://benbedphar.org/webinars/. The third webinar took place on July 7th on “NRF2 and metabolic disease”, with the leading experts Drs. Elke H. Heiss, Gerasimos Sykiotis, and Nesrin Kartal Ozer. The webinar gathered over 80 participants. The next webinar is scheduled for October 2022.
In May 30th, BenBedPhar participated in a MDPI webinar with title “Role of NRF2 in Disease: Novel Molecular Mechanisms and Therapeutic Approaches II”. The webinar was chaired by Dr. ‘Isabel Lastres Becker and the BenBedphar speakers were Profs. Albena Dinkova-Kostova and Antonio Cuadrado. See more details at sciforum.
Publication in Special issues
1) Bench to bedside transition for pharmacological regulation of NRF2 in non-communicable diseases, in Free Radials Biology and Medicine, 2) Transcription Factor NRF2, in Antioxidants, 3) DNA Damage, Oxidative Stress and Human Disease, in International Journal of Molecular Sciences, 4) Role Of Nrf2 In Disease: Novel Molecular Mechanisms And Therapeutic Approaches II, in Biomolecules. In these issues, 22 articles related to NRF2 have been published so far, of which 13 are authored by members of our network. See: https://benbedphar.org/special-issues/.
BebBedPhar Open day
In July 6th, our first BenBedPhar open day took place at “Victor Babes” National institute of Pathology, Bucharest, Romania. The chair, Antonio Cuadrado and the vice chair, Gina Manda, presented our action’s goals, challenges, organizational structures, and activities, using a template model that had been elaborated by our Dissemination officer, Aleksandra Buha. There were close to 50 participants.
Antonio Cuadrado
Chair of COST Action 20121, BenBedPhar
Autonomous University of Madrid
Comments from the Working groups
“Tricking” KEAP1 with small molecules with a binding mode similar to that of the ETGE-NRF2 peptide
The multitude of targets of even the most potent and selective electrophilic NRF2 activators (Liby and Sporn, 2012) has sparked a growing interest in specifically targeting KEAP1 with non-electrophilic protein-protein interaction (PPI) inhibitors. Indeed, using VirtualFlow, an open-source platform that enables chemical space screening on an ultra-large scale, approximately 1.3 billion compounds have been screened against the Kelch domain of KEAP1, the site of NRF2 interaction (Gorgulla et al. 2020). Binding of such PPI inhibitors to KEAP1 utilizes a molecular mechanism leading to NRF2 activation known as “Hinge & Latch”, where the DLGex-binding motif of NRF2 dissociates from KEAP1 as a latch, while the ETGE motif of NRF2 remains attached to KEAP1 as a hinge (Horie et al. 2020). Overall, this limits the availability of free KEAP1 for binding newly-synthesized NRF2, which leads to accumulation of the transcription factor, followed by nuclear translocation and enhanced transcription of its target genes. Recently, the use of a structure-based ligand design approach uncovered a new class of phenyl bis-sulfonamide KEAP1 : NRF2 PPI inhibitors (Georgakopoulos et al. 2022). Unexpectedly, the high-resolution co-crystal structure of one such compound, 2,2′-((2-(Benzyloxy)-1,4-phenylene)bis(((4-methoxyphenyl)sulfonyl)azanediyl))diacetic acid, bound to the Kelch domain of KEAP1 revealed an unusual binding mode which closely resembles the KEAP1 : ETGE-NRF2 peptide complex (Figure 1). This is in contrast to the binding mode of other known aryl bis-sulfonamides, which preferentially disrupt the binding of KEAP1 to the DLG motif of NRF2. As typical for most of the KEAP1 : NRF2 PPI inhibitors known to date, the potency of these compounds in activating NRF2 in cells (and presumably also in vivo) needs much improvement. Nonetheless, this unique binding mode of the new series of compounds suggests the possibility to use small molecules that bind to the Kelch domain of KEAP1 as the endogenously-occurring peptide motif does, and thus offer an alternative strategy for future drug optimization.
- Georgakopoulos N, Talapatra S, Dikovskaya D, Dayalan Naidu S, Higgins M, Gatliff J, Ayhan A, Nikoloudaki R, Schaap M, Valko K, Javid F, Dinkova-Kostova AT, Kozielski F, Wells G. Phenyl Bis-Sulfonamide Keap1-Nrf2 Protein-Protein Interaction Inhibitors with an Alternative Binding Mode. J Med Chem. 2022 May 26;65(10):7380-7398.
- Gorgulla C, Boeszoermenyi A, Wang ZF, Fischer PD, Coote PW, Padmanabha Das KM, Malets YS, Radchenko DS, Moroz YS, Scott DA, Fackeldey K, Hoffmann M, Iavniuk I, Wagner G, Arthanari H. An open-source drug discovery platform enables ultra-large virtual screens. Nature. 2020 Apr;580(7805):663-668.
- Horie Y, Suzuki T, Inoue J, Iso T, Wells G, Moore TW, Mizushima T, Dinkova-Kostova AT, Kasai T, Kamei T, Koshiba S, Yamamoto M. Molecular basis for the disruption of Keap1-Nrf2 interaction via Hinge & Latch mechanism. Commun Biol. 2021 May 14;4(1):576.
- Liby KT, Sporn MB. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev. 2012 Oct;64(4):972-1003.
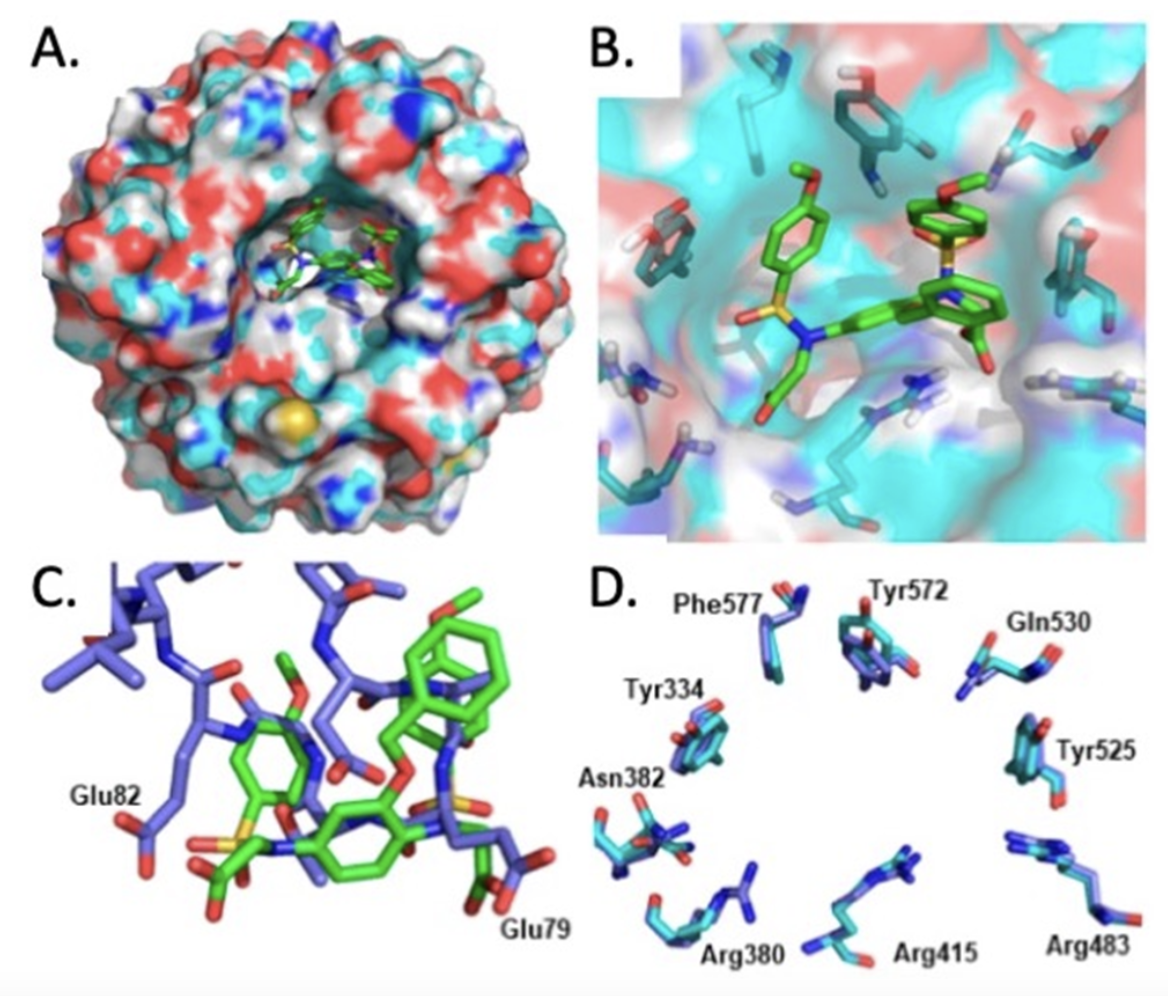
Figure 1: (A, B) Bound conformation of the phenyl bis-sulfonamide 2,2′-((2-(benzyloxy)-1,4-phenylene)bis(((4-methoxyphenyl)sulfonyl) azanediyl))diacetic acid co-crystallized with the KEAP1 Kelch domain; (C) overlay of the bound conformation of the same compound and the ETGE peptide bound to KEAP1 (PDB entry 2FLU); (D) comparison of the KEAP1 Kelch domain binding pocket in the compound co-crystal structure (cyan) and the 2FLU ETGE peptide co-crystal (blue). Figure adapted from: Georgakopoulos et al, 2022
Albena Dinkova-Kostova
WG2 leader
University of Dundee
UK
Personalized redox medicine in inflammatory bowel diseases
Inflammatory bowel diseases (IBD) constitute chronic, immune-mediated diseases of the gastrointestinal tract, among which two subtypes are usually distinguished: Crohn’s disease (CD) and ulcerative colitis (UC). IBD is characterized by complex, multifactorial etiology, consisting of an interplay between genetic susceptibility, gut microbiome disturbances, immunological alterations, and lifestyle-, dietary- and environmental factors. Clinical symptoms of IBD include, among others, abdominal pain, diarrhea, fatigue, and also extraintestinal manifestations (e.g. arthritis), but these symptoms are often nonspecific and very heterogeneous across patients. As such, patients with IBD frequently suffer from long-lasting subclinical disease activity that is difficult to monitor, to treat and to predict. This explains the fact that a major part of clinical care for these patients is dedicated to accurate disease activity monitoring to enable early-onset medical treatment. Among various pathophysiological processes that play a role, oxidative stress is considered a key mechanism driving the onset and progression of IBD. Oxidative stress is defined as an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage. Patients with IBD are marked by disturbances of redox signaling pathways which can be minimally invasively quantified by measuring components of the systemic redox status, i.e. redox biomarkers. Many of these biomarkers have been investigated in the context of IBD for their clinical utility, but as of yet it is not always clear what all these biomarkers exactly represent and what their relative contributions are in relation to whole-body redox status. Therefore, the discovery and validation of redox biomarkers for IBD need optimization by prioritizing central regulatory hubs, developing redox metabolomics approaches, and establishing integrative biomarkers that combine read-outs of multiple redox-regulated events. To make further progress in this context, there is an increased advocacy of personalized redox medicine approaches, which aim to selectively inhibit pathologic overproduction or -expression of specific enzymatic sources of reactive species on individual patient level, without affecting their physiological functions. To achieve this, a finer understanding of disrupted redox signaling in IBD is further required, together with renewed terminology and clinical integration of existing biological profiling (“omics”) technologies. Considering that redox-targeted pharmacological interventions are emerging in the context of IBD (currently in early phase clinical trials), it would be even more important to strive for these aims. These pharmacological interventions are aimed at activating endogenous antioxidant defense systems and viable therapeutic options for restoring redox homeostasis. Two main targets hold promise in this regard: the nuclear factor erythroid 2-derived factor 2 (NRF2) and Kelch-like ECH-associated protein 1 (KEAP1) pathway and the hypoxia-inducible factor-1α (HIF1α) pathway. Such redox-directed pharmacological interventions may offer new opportunities to break the therapeutic ceiling in IBD.
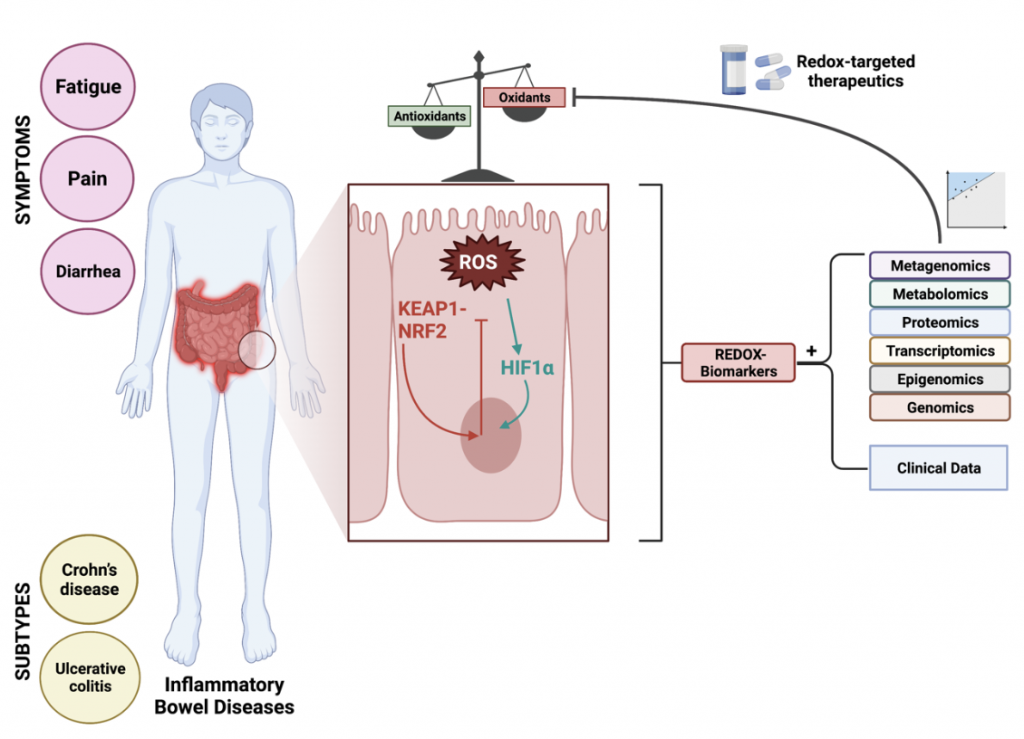
Figure 2. Redox-targeted therapeutics in personalized therapy of inflammatory bowel disease
Arno Bourgonje
Harry Van Goor
WG3 members
University Medical Center Groningen
Netherlands
Christina Morgenstern
WG3 Co-Leader
Karl-Franzens Universität
Austria
NRF2 in the thyroid
Thyroid hormones are the main regulators of the basal metabolic rate. Work by our group and others over the last years has shown that KEAP1/NRF2 signaling has pleiotropic roles in the thyroid gland. These roles include a direct positive transcriptional effect on the gene that encodes thyroglobulin, the thyroid hormone precursor; this is balanced by a negative effect on the iodination of thyroglobulin, which is a critical step in the synthesis of thyroid hormones. NRF2 also mediates transcriptional antioxidant responses in thyroid cells, and it interacts with the selenoprotein system to protect against the development of autoimmune thyroiditis. These findings suggest that NRF2-modulating compounds might be useful in the prevention or treatment of thyroid diseases. On the other hand, NRF2 is commonly activated in thyroid carcinomas, where it mediates protective antioxidant responses and favors the survival of cancer cells. Moreover, constitutive activation of NRF2 due to decreased Keap1 expression leads to thyroid enlargement (goiter) in humans with germline loss-of-function mutations in the KEAP1 gene as well as in mice with decreased expression of KEAP1 (Keap1 knock-down mice). In humans, goiter was accompanied by the formation of multiple thyroid nodules (multinodular goiter), while in mice, a mild form of decreased thyroid function (hypothyroidism) was observed. These latter observations raise concern about possible thyroidal side-effects of NRF2-activating compounds. Nevertheless, available evidence from a clinical trial of sulforaphane for detoxification in China, and from a clinical cohort of patients treated with dimethyl fumarate for multiple sclerosis in Switzerland, does not indicate a negative impact of these two NRF2 activators on the thyroid. However, effects on thyroid structure have not been assessed systematically and prospectively using clinical examination or ultrasound. Thus, further studies on the roles of KEAP1/NRF2 signaling in thyroid biology and pathophysiology are warranted to document the thyroidal safety of NRF2 modulators and to realize the translational potential of NRF2 as a drug target for the prevention and treatment of thyroid diseases. Finally, because thyroid hormones regulate metabolism, studies of metabolism using in vivo models with altered NRF2 activity should also systematically document the thyroid function of their respective experimental groups.
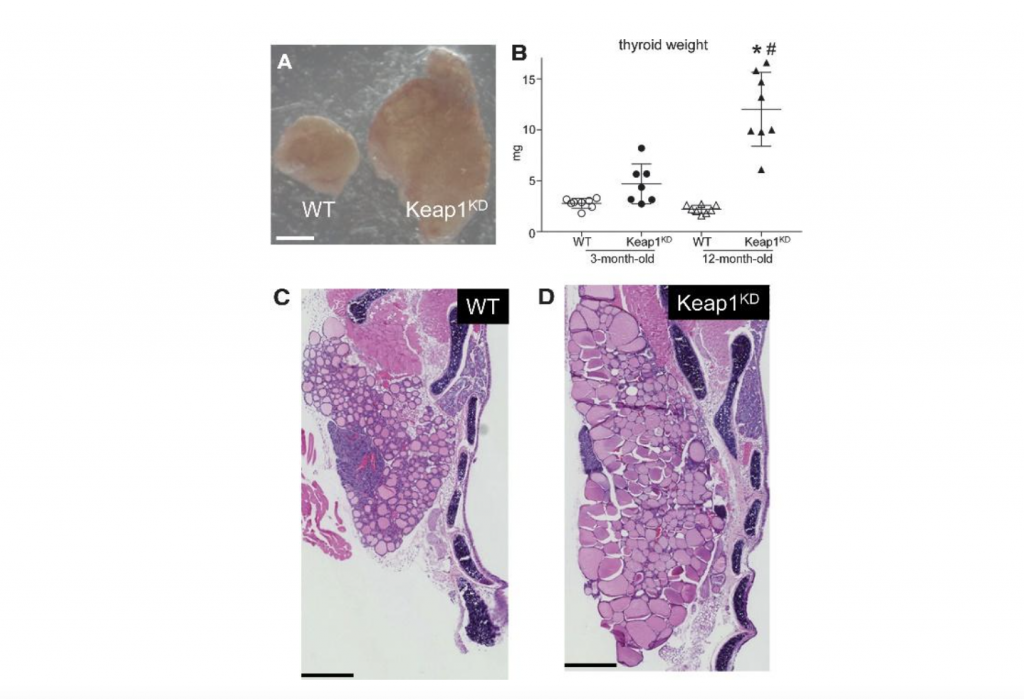
Figure 3. Goiter phenotype in KEAP1 hypomorphic (Keap1KD) mice. (A) Photograph of a single thyroid lobe from a 12-month-old male WT control mouse and a Keap1KD mouse of the same age. Scale bar indicates 0.5 mm. (B) Weight of fresh thyroid (both lobes) in male WT and Keap1KD mice. Data are shown as mean ±standard deviation. *p<0.05 versus 12-month-old WT; #p<0.05 versus 3-month-old Keap1KD. (C, D) H&E stain of thyroid lobes in 12-month-old male WT (C) and Keap1KD (D) mice. Scale bar indicates 0.5 mm. H&E, hematoxylin and eosin; Keap1KD, Keap1 knock-down; WT, wild-type. Adapted from Ziros et al. 2021 Thyroid 31: 23-35, published by our group in Open Access.
Gerasimos Sykiotis
WG2,3 member
Lausanne University Hospital
Switzerland
NRF2: a patent review (last three years)
Following the ongoing clinical trials assessment led by Fernando Antunes (WG4 co-leader), a patent search is being performed for upcoming NRF2 technologies. Using WIPO (World Intellectual Property Organization) and Espacenet databases, all patents in the last three years specifying “NRF2”, “Nuclear factor erythroid 2-related factor 2 ”, or “nuclear factor erythroid-derived 2-like 2”, in the title or abstract had been collected (n=403). After duplicated removal, a CSV file containing 223 distinct patents was generated, which includes key data: patent title, a link to the original documents, inventors, publication dates, applicants as well as the abstract of the patent. The advance of AI translation capabilities of the search engines enables to cover patent submitted not only in English (e.g., Chinese) and therefore reflect the overall progression in the field. Based on our preliminary observations, the majority of innovations present new NRF2 activators, of which natural extracts or isolated compounds are prominent. In addition, a combination therapy (NRF2 modulators that are supplemented with additional API with additive or synergistic action) had been submitted by several groups. More than ten inventions describe NRF2 inhibitors (direct or indirect), needed in the treatment of various cancer types with abnormal activation of the NRF2 cascade. Lastly, several new methodologies to screen for new NRF2 modulators or to identify NRF2-based biomarkers had been introduced. Besides the technological progression, the database can help in targeting companies, already with NRF-2-based technologies, for joint academic-industrial projects. The combined patent search and the clinical trial data will enable the assessment of market direction as well as the upcoming cutting-edge technologies in the field.
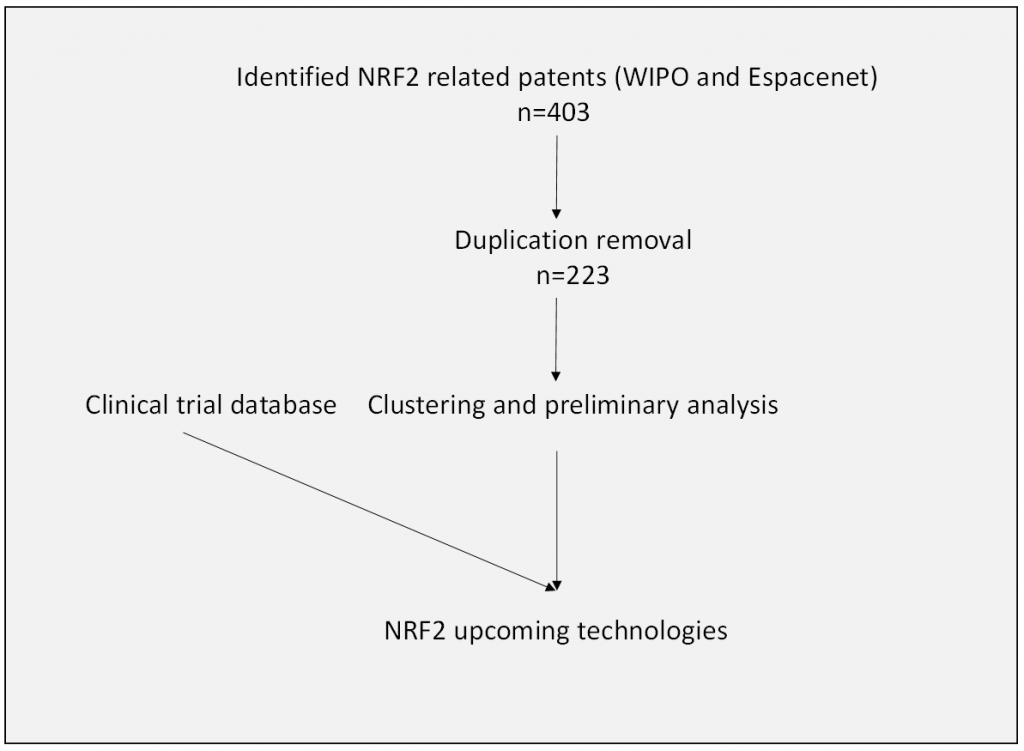
Figure 4. Landscape of NRF2-related patents in the last three years
Guy Cohen
WG4 Co-leader
The Dead-Sea & Arava Science Center
Israel
The origin of NRF2
The KEAP1-NRF2 pathway is an evolutionary conserved strategy to deal with oxidative stress. But how far back in evolution do we need to go in order to find NRF2-like ancestor proteins? To answer this question, we need to go back approximately 2,35 billion years, to the Great Oxidation Event, when the oxygen levels on Earth started to rise. This event led to dramatic metabolic changes in the living organisms and an adaptation to the potentially harmful production of reactive oxygen species (ROS). According to the work by Gacesa et al, the first NRF2 orthologues appeared around 1.5 billion years ago, during the Proterozoic in fungi, and from that point onwards several NRF2 orthologues have diverged and can now be found across different species, including ascidians, sea urchins, octopus and several model organisms, such as the worm Caenorhabditis elegans, the fruit fly Drosophila melanogaster or zebrafish. Comparison between NRF2 proteins across different species has shown that the essential DNA binding region, the cap’n’collar (CNC)–basic region-leucine zipper (bZIP), is extremely conserved. Moreover, the presence of the CNC-bZIP region allows the binding to antioxidant responsive element (ARE)-like structures and the transcriptional regulation of several genes coding for proteins involved in the oxidative stress response. In contrast, other domains, such as the animo-terminal Neh2 domain, required for KEAP1 binding, are only present in some species. The presence of the Neh2 domain is thought to be due to another rise in atmospheric oxygen levels, approximately 550 million years ago, which represented a strong evolutionary pressure and led to the recruitment of KEAP1 as part of an efficient protective system against ROS. Overall, understanding the evolution of the KEAP1-NRF2 pathway may help us to dissect out the different mechanisms where this pathway is implicated, using the most adequate animal models of disease.
- Fuse, Y., and Kobayashi, M. (2017). Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Mol. A J. Synth. Chem. Nat. Prod. Chem. 22.
- Gacesa, R., Dunlap, W.C., Barlow, D.J., Laskowski, R.A., and Long, P.F. (2016). Rising levels of atmospheric oxygen and evolution of Nrf2. Sci. Reports 2016 61 6, 1–5.
- Maher, J., and Yamamoto, M. (2010). The rise of antioxidant signaling—The evolution and hormetic actions of Nrf2. Toxicol. Appl. Pharmacol. 244, 4–15.
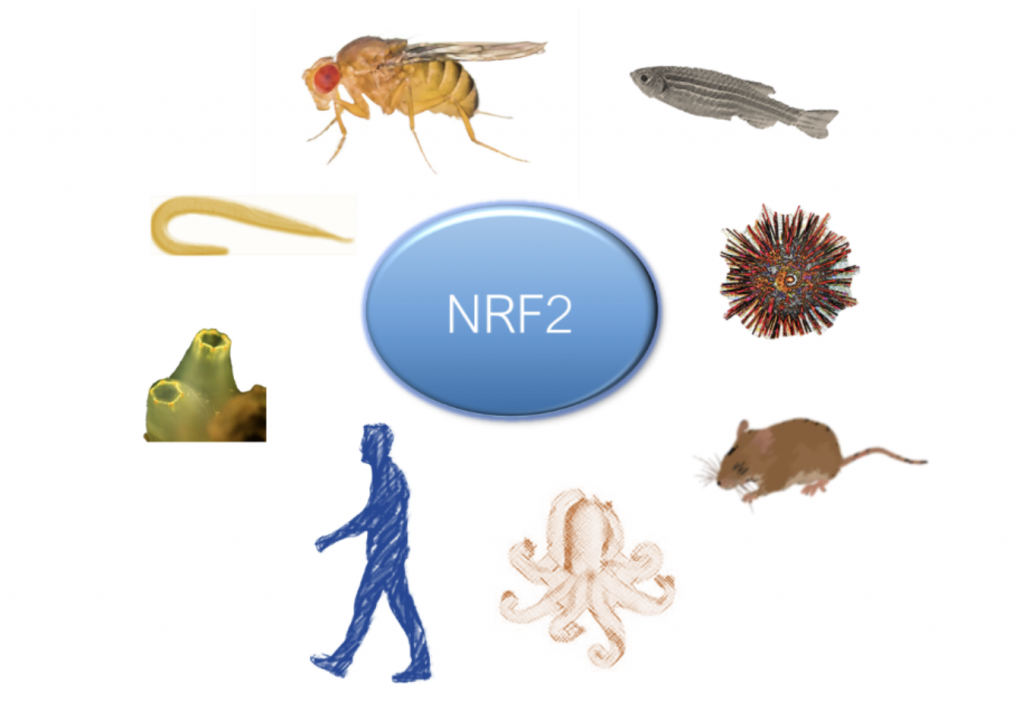
Figure 5. The KEAP1 NRF2 pathway is an evolutionary conserved strategy to deal with oxidative stress
Ana Rita Carlos
WG5 Co-Leader
University of Lisbon
Portugal
NRF2 fights environmental stressors
Environmental risk factors account for a major part of non-communicable diseases. Among these environmental stressors are traffic noise, air pollution, chemical agents such as heavy metals and pesticides, ultraviolet radiation (UVR) and mental stress but also lifestyle factors such as smoking. As discussed by Bayo Jimenez et al. and Daiber, oxidative stress and inflammation are key players in the molecular pathomechanisms of environmental pollutants and stressors, which can be efficiently prevented by activation of the nuclear factor erythroid 2-related factor 2 (NRF2) (Bayo Jimenez, Frenis et al. 2022). A great number of studies support the protective actions of NRF2 and its downstream pathways against different environmental stressors (see scheme below). NRF2-dependent heme oxygenase-1 induction, including the interaction with BACH1 system, is highly protective against environmental stressors, mainly by producing the vasodilator and anti-atherosclerotic molecule carbon monoxide as well as the antioxidants biliverdin and bilirubin. Since all environmental stressors largely affect the circadian rhythm in a negative fashion, the known beneficial interactions of NRF2 with the circadian clock may play a key role in its mitigation of environmental adverse health effects. Our review article identified and discussed a plethory of NRF2 activators in relation to environmental stressor-induced health side effects. Based on these considerations, nutraceuticals that activate NRF2 may be suitable to prevent the negative effects of the environment on our health.
Bayo Jimenez, M. T., K. Frenis, O. Hahad, S. Steven, G. Cohen, A. Cuadrado, T. Munzel and A. Daiber (2022). “Protective actions of nuclear factor erythroid 2-related factor 2 (NRF2) and downstream pathways against environmental stressors.” Free Radic Biol Med 187: 72-91.
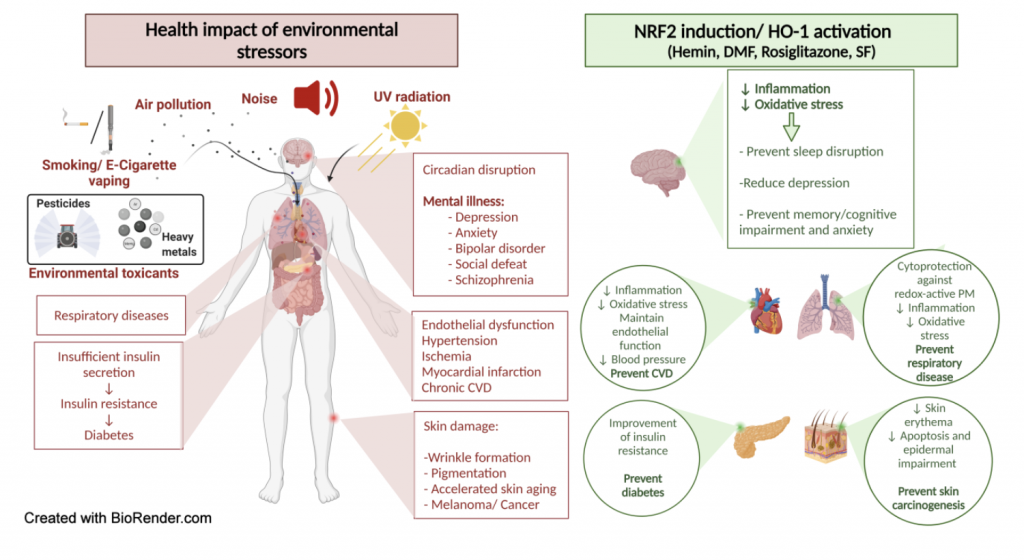
Figure 6. Protective actions of NRF2 against environmental stressors
Andreas Daiber
WG2,3,5 member
University Medical Center of the Johannes Gutenberg University Mainz
Germany
Invited article
In January 2020, I was approached by Dr. Albert Wright. He is a retired British researcher based in Grenoble, France, that had been suffering from with Parkinson’s disease (PD) for more than 2 years at that time. He was looking for ways to slow or reverse his disease and, based on the available literature on the NRF2 activator sulforaphane and PD, he started to take an infusion of broccoli seeds, rich in glucosinolates and isothiocyanates. A careful self-analysis of his disease led him to conclude that the sulforaphane-rich tea improved several PD symptoms. Unfortunately, he could not engage clinicians in the analysis of his disease progression and, due to lack of a highly controlled trial, based in school pharmacology and medicine, our letter correspondence cooled down. Recently, Albert contacted me again, as well as Dr. Albena Dinkova-Kostova, world expert in NRF2-related pharmacology, and Dr. Jed Fahey, expert in extraction of sulforaphane from Cruciferous vegetables at John Hopkins University. In these years he had organized a support group with several PD patients that were also taking a sulforaphane-rich infusion. While a carefully controlled trial is still necessary, Albert’s essay depicts a crucial problem in the NRF2 field, which is that most NRF2 activators are of natural origin and difficult to be protected with strong patents by biopharmaceutical companies. This fact represents a significant restrain in development of NRF2-related therapeutics in diseases such as PD. In other words, pharmacological research is not always focused on patients but on investors.
Antonio Cuadrado
Chair of COST Action 20121, BenBedPhar
Autonomous University of Madrid
Reflections of a scientist with Parkinson’s Disease by Dr. Albert Wright (2022)
This is the story of the first 4 years of a scientist’s journey with Parkinson’s disease. It begins with the diagnosis and the neurologist’s affirmation: “The cause of Parkinson’s Disease is unknown… “, “that’s why there are no drugs to slow or stop Parkinson’s disease”. That was a very bad start. After consulting publications by the world’s leading fundamental research scientists, it became clear that these experts know quite enough about the causes of Parkinson’s disease to identify targets for drugs to slow Parkinson’s disease. People with Parkinson’s are not being told the truth about their disease.
Join me on this journey. You will meet some of the experts who helped me build a model describing the events occurring in the early stages of Parkinson’s disease and develop a method based on a plant-based molecule to combat these events. I have been using and improving this method since the beginning of 2020 with good results and sharing progress with a small group of People with Parkinson’s. All People with Parkinson’s are now invited to join our group and learn more about the method.
You will also learn how the Pharmaceutical Industry fails to address the most important steps driving the progression of Parkinson’s Disease and concentrates uniquely on symptomatic therapies. People with Parkinson’s will understand why no disease-modifying therapies will be developed by the Pharmaceutical Industry in their lifetime.
Finally, fundamental research scientists are invited to join our initiative “STOP”, Scientists Take On Parkinson’s. “STOP” is dedicated to encouraging Fundamental Research Scientists to occupy the research space left vacant by the Pharmaceutical Industry relating to the causes of Parkinson’s Disease. A guiding principle of STOP will be that non-scientific criteria such as those that typically apply to plant-based substances for drug development will not apply in “STOP”.
Reflections … takes the form of a presentation. It is a PDF File which you can download it here:
Hot from Pubmed
Glutaminase inhibition effect in CD8 T cell activation
Glucose and glutamine in the tumor microenvironment (TME) are essential for the development and activation of effector T cells that exert antitumor function. Immunotherapy potentiates T cell antitumor function. However, a significant proportion of patients does not benefit from this treatment. In patients with KRAS-mutant lung adenocarcinoma, KEAP1 and STK11/Lkb1 co-mutations are associated with impaired response to immunotherapy. To investigate the metabolic and immune microenvironment of KRAS-mutant lung adenocarcinoma, the authors generated murine models that reflect the KEAP1 and STK11/Lkb1 mutational landscape in these patients. Degradation of glutamine into glutamate is promoted by the NRF2 target gene glutaminase and in this work, it was showed that increased glutamate abundance in the Lkb1-deficient TME is associated with CD8 T cell activation in response to anti-PD1. Moreover, combined treatment with the glutaminase inhibitor CB-839 inhibited clonal expansion and activation of CD8 T cells, negatively impacting CD8 T cells activated by anti-PD1 immunotherapy.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35504291/
A CRISPR screen identifies redox vulnerabilities for KEAP1/NRF2 mutant non-small cell lung cancer
The redox regulator NRF2 is hyperactivated in a large percentage of non-small cell lung cancer (NSCLC) cases, which is associated with chemotherapy and radiation resistance. However, NRF2 control in ROS detoxification is complex and redundant. To investigate redox vulnerabilities, Jiang and collaborators conducted a CRISPR-Cas9-based negative selection screen for antioxidant enzyme genes whose loss sensitized cells to sub-lethal concentrations of the superoxide (O2•-)-generating drug β-Lapachone. Besides identifying expected hits in the pentose phosphate pathway, the thioredoxin-dependent antioxidant system and glutathione reductase, the mitochondrial superoxide dismutase 2 (SOD2) was identified as one of the top hits. Importantly, SOD2 loss enhanced the efficacy of β-Lapachone due to loss of iron-sulfur protein function, loss of mitochondrial ATP maintenance and deficient NADPH production. Moreover, inhibition of mitochondrial electron transport activity sensitized cells to β-Lapachone, demonstrating a therapeutic potential in the modulation of ROS sensitivity.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35667246/
Guidelines for measuring ROS and oxidative damage in cells and in vivo
Researchers from different fields are now studying reactive oxygen species (ROS) due to their involvement in different pathologies. Indeed, there are many assays and commercial kits available, but their use and interpretation are challenging and open to artefacts, specially by those working outside the area. As such, a consensus statement was created highlighting problems that can arise with many commonly used approaches for measurement of ROS and oxidative damage, along with the proposal of guidelines for best practice.The goal is that these strategies will be useful to those who find their research requiring assessment of ROS, oxidative damage and redox signalling in cells and in vivo.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35760871/
NRF2 pathway activation attenuates ageing-related renal phenotypes
Oxidative stress is strongly related to age-induced functional decline in cells and tissues. The KEAP1-NRF2 system plays a central role in the regulation of redox balance, and NRF2 activation exerts antiageing effects by controlling oxidative stress in aged tissues. α-Klotho was identified as an ageing suppressor protein based on the premature ageing phenotypes of its mutant mice, and its expression is known to gradually decrease during ageing. Because α-klotho has been shown to possess antioxidant function, ageing-related phenotypes of α-klotho mutant mice seem to be attributable to increased oxidative stress at least in part. To examine whether NRF2 activation antagonizes ageing-related phenotypes caused by α-klotho deficiency, α-klotho-deficient (Kl-/-) mice was crossed with a Keap1-knockdown background, in which the NRF2 pathway is constitutively activated in the whole body. NRF2 pathway activation in Kl-/- mice extended the lifespan and improved ageing-related renal phenotypes with elevated expression of antioxidant genes accompanied by an oxidative stress decrease, namely through the inhibition of oxidative stress accumulation, fibrosis, calcification and apoptosis. Thus, NRF2 is demonstrated to have an antiageing function by attenuating renal pathologies originating from α-klotho deficiency.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35137128/
NRF2 activation mediates the anti-inflammatory properties of a subset of over-the-counter and prescription NSAIDs
Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit cyclooxygenase (COX) enzymes and are ubiquitously used for their anti-inflammatory properties. However, COX inhibition alone fails to explain numerous clinical outcomes of NSAID usage. Screening commonly used NSAIDs in primary human and murine myeloid cells demonstrated that NSAIDs could be differentiated by their ability to induce growth/differentiation factor 15 (GDF15), independent of COX specificity. The group of Eisenstein demonstrated that NSAID-mediated GDF15 induction was dependent on the activation of NRF2 in myeloid cells and that sensing by KEAP1 was required for NSAID activation of NRF2 and subsequent anti-inflammatory effects both in vitro and in vivo. This work highlights a noncanonical NRF2-dependent mechanism of action for the anti-inflammatory activity of a subset of commonly used NSAIDs.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35588739/
Neuroprotective effect of the Nrf2 in spinal cord injury
Spinal cord injury (SCI) is associated with chronic neuroinflammation and redox imbalance. Oxidative stress is one of the main hallmarks of secondary injury of SCI which is tightly regulated by nuclear factor E2-related factor 2/antioxidant response element (Nrf2/ARE) signaling. Moreover, miRNA expression is temporally altered after SCI, and these alterations may have a critical effect on the pathogenesis of SCI by regulating inflammation, demyelination, and apoptosis. The work from Ebrahimy and collaborators suggests that astrocytic hyperactivation of Nrf2 exert neuroprotective effects at least in part through the upregulation of miRNA145-5p, a negative regulator of astrocyte proliferation.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35750202/
Nrf2 protects against radiation-induced oral mucositis via antioxidation and keratin layer thickening
Radiation-induced oral mucositis is one of the most common adverse events in radiation therapy for head and neck cancers, but treatments for oral mucositis are limited to palliative and supportive care. Given previous reports on Nrf2 cytoprotection against oxidative and electrophilic stresses and regulation of keratin layer thickness in mouse tongues, Wakamori and collaborators demonstrated that Nrf2 activation by genetic Keap1 knockdown alleviated radiation-induced DNA damage by increasing antioxidation. In agreement with the genetic Nrf2 activation model, a Nrf2 inducer prevented irradiation damage to the tongue epithelium.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35753588/
Fisetin Attenuated Oxidative Stress-Induced Cellular Damage in ARPE-19 Human Retinal Pigment Epithelial Cells Through Nrf2-Mediated Activation of Heme Oxygenase-1
Oxidative stress-induced retinal disorders are thought to play an important role in the pathogenesis of irreversible vision loss-related diseases, including retinitis pigmentosa, diabetic retinopathy and age-related retinal degeneration. Fisetin is a bioactive flavonol, present in fruits such as strawberries and apples, and is known to act as a potent free radical scavenger. Its effect in human retinal pigment epithelial (RPE) cells injured with hydrogen peroxide was investigated. Indeed, fisetin alleviated mitochondrial dysfunction and enhanced phosphorylation and nuclear translocation of Nrf2, which was associated with increased expression and activity of heme oxygenase-1 (HO-1). Accordingly, fisetin protective effects were reversed with a HO-1 inhibitor, suggesting that Nrf2-mediated activation of antioxidant enzyme HO-1 may play an important role in the ROS scavenging activity of fisetin in retinal pigment epithelial cells.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35784747/
Modulating Nrf2 as part of a multifaceted therapeutic approach for Alzheimer’s disease
The etiology of Alzheimer’s disease (AD) includes amyloid β (Aβ) amyloidosis, metal–Aβ42 complex formation, ROS, oxidative stress, mitochondrial damage, and neuroinflammation. Thus, multifunctional modulators were designed by integrating pharmacophores for metal chelation, antioxidant and anti-inflammatory properties, and modulation of Aβ42 aggregation on the naphthalene monoimide (NMI) scaffold. Treatment with one of the synthesized molecules synergistically modulated metal-independent and -dependent amyloid toxicity, quenched the ROS and reduced oxidative stress, accompanied by the reduced the translocation of Nrf2 to the nucleus in neuronal cells. This work further supports the importance of modulating Nrf2 in AD therapeutics.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35759686/
Carbocysteine modulation of Nrf2/HO-1 in the treatment of ulcerative colitis
Current therapies for ulcerative colitis (UC), an inflammatory bowel disease with multifaceted pathophysiology, lack efficacy. It has been well documented that modulating the Nrf2/NFκB is a promising therapeutic target in inflammation. Moreover, carbocisteine is a mucoregulatory medication and its efficacy in COPD was found to be more closely related to its antioxidant and anti-inflammatory properties. In this work, it was investigated the potential coloprotective role of carbocisteine in acetic acid-induced colitis in rats. The results revealed that carbocisteine attenuated colon shortening and augmented colon antioxidant defense mechanisms via upregulating catalase and HO-1 enzymes. The myeloperoxidase activity was suppressed indicating inhibition of the neutrophil infiltration and activation. Consistent with these findings, carbocisteine boosted Nrf2 expression along with NFκB inactivation. Consequently, carbocisteine downregulated the proinflammatory cytokines IL-6 and TNF-α and upregulated the anti-inflammatory cytokine IL-10. Concomitant to these protective roles, carbocisteine displayed anti-apoptotic properties as revealed by the reduction in the Bax: BCL-2 ratio. In conclusion, carbocisteine inhibited oxidative stress, inflammatory response, and apoptosis in acetic acid-induced UC by modulating the Nrf2/HO-1 and NFκB interplay in rats. Therefore, the current study provides a potential basis for repurposing a safe and a commonly used mucoregulator for the treatment of UC.
Access to the original article: https://pubmed.ncbi.nlm.nih.gov/35754464/
Joana Miranda
University of Lisbon
Portugal


