Thursday, February 8, 2024
- 9:00 – 11:00 Scientific Session I: Pharmacological targeting of NRF2 (I) Chair: Ioannis Trougakos
Electrophilic NRF2 Activating Drugs: Biomarkers and Specificity
Thomas W. Kensler
Affiliate Investigator, Fred Hutchinson Cancer Center
Professor Emeritus, Johns Hopkins University
Hundreds of compounds, mainly natural products or their congeners, have been described as inducers of NRF2 signaling. Most, while effective, are not potent. Five molecules with a range of inducer potencies – dimethyl fumarate; bardoxolone methyl; omaveloxolone; oltipraz; and sulforaphane – have been utilized in clinical trials under the guise of “NRF2 inducer”. However, all are electrophilic molecules with a potential capacity to touch multiple cysteine sensors in the cell, leading to a potential myriad of signaling responses. Pharmacodynamic biomarkers reflecting action on the NRF2 system have been observed in clinical trials with all these agents, but the signals are usually variable and of small magnitude. No evidence affirms a direct role of NRF2 induction in the clinical outcomes measured. By contrast, drugs studied in wild-type and Nrf2 knockout mice exhibit consistent, albeit incomplete, loss of efficacy with disruption of the putative target. Contributions by other targets nonetheless remain as confounders. Using a highly refined disruption of the molecular target, C151S KEAP1 mutant mice, the efficacy of bardoxolone methyl to induce NRF2 signaling is abolished as is protection against T-cell mediated immune hepatitis following Concanavalin A challenge. Thus, bardoxolone methyl appears to be an NRF2-specific inducer at modest (but effective) doses in vivo. Target specificity is a function of dose – higher dose, off-target actions may provide an advantage (broader efficacy) or disadvantage (toxicity). Such a consideration is also of relevance to the ongoing development of PPIs affecting NRF2-KEAP1 interactions, which while surmised to exhibit high target specificity may displace multiple signaling proteins. Supported by NIH R35 CA197222.

Thomas W. Kensler, Thomas Kensler obtained his doctorate at MIT and trained as a postdoctoral fellow at the University of Wisconsin and at the National Cancer Institute. After 30 years on the faculty of the School of Public Health at Johns Hopkins, he moved his primary appointment to the School of Medicine, University of Pittsburgh in 2010. He moved his lab to the Fred Hutchinson Cancer Center in 2018 and retired in early 2024. He is now a Professor Emeritus at Johns Hopkins. His more recent goals have been to evaluate whether broccoli sprout beverages or broccoli-based dietary supplements rich in sulforaphane can enhance the detoxication of environmental (e.g., aflatoxin, air pollutants) and endogenous (e.g.,estrogen) carcinogens to reduce the levels of associated DNA damage and offer a potentially effective and frugal approach for the prevention of several forms of cancer. Triggering the KEAP1-NRF2 adaptive stress response pathway serves as a key molecular target for these efforts. He has received numerous awards including the AACR-American Cancer Society Award for Excellence in Cancer Epidemiology and Prevention, Society of Toxicology Translational Impact Award, the National Friendship Award, Beijing, China’s highest award for foreign civilians, and an Outstanding Investigator Award from the NCI. He has published over 350 research articles.
Developing therapies to target NRF2
Karen T. Liby
To test the hypothesis that modulating inflammation could be useful for the prevention or treatment of cancer, Dr. Michael Sporn started collaborating with two chemists, Drs. Gordon Gribble and Tadashi Honda, at Dartmouth Medical School on a new drug discovery program with oleanolic acid as the starting material. This program resulted in the synthesis and biological testing of several hundred novel synthetic oleanane triterpernoids. These compounds not only inhibited inflammation in vitro and in vivo but also are potent activators of the NRF2 cytoprotective pathway. Although initial clinical trials of the first lead triterpenoid CDDO-methyl ester (bardoxolone methyl) were in cancer, unexpected results in this first clinical trial culminated in multiple clinical studies in diseases ranging from chronic kidney disease to pulmonary arterial hypertension. Despite numerous setbacks during the clinical development of the oleanane triterpenoids, omaveloxolone has been approved for the treatment of Friedreich’s ataxia. Challenges addressed during the development of the oleanane triterpenoids are relevant to new programs targeting the NR2 pathway.

Karen Liby is a Professor of Medicine and the Lois and Sid Eskenazi Chair in Hematology/ Oncology at the Simon Comprehensive Cancer Center. The goal of her research is to identify new compounds that inhibit inflammation or modulate the immune system for the prevention and treatment of cancer. Her lab has completed efficacy and mechanistic studies with clinically relevant drugs including synthetic oleanane triterpenoids, RXR agonists, PARP inhibitors, and bromodomain inhibitors. Dr. Liby earned a B.S. in biology and history from Hillsdale College and a Ph.D. in cellular and molecular biology from the University of Cincinnati. She was the recipient of the Wilson S. Stone Memorial Award from the M.D. Anderson Cancer Center and an Early Promise of Research Excellence Award from Michigan State University.
Experimental translational research and development of new therapies.
The role of NASCE / UEMS: Current challenges and future perspectives
Apostolos E. Papalois
Secretary General NASCE / UEMS Executive Board.
Visiting Professor School of Medicine,
National and Kapodistrian University of Athens.
President Scientific Council SUBRE
School of Medicine Aristotle University Thessaloniki.
The Nobel Prizes for Medicine and Physiology, such as other relative Nobel, for example Chemistry, is an important indicator, which shows as the importance of the use of laboratory animals, in medical research and its applications. Over 70% of the above mentioned Nobel Prizes, have used immediately or indirectly, animal models for the announcement of their significant discoveries. But it is not only about that. These Prizes are furthermore the most significant prove for the importance of cooperation of the scientific specialties not only in Medicine, which is a huge scientific family, but also in many other scientific fields. For example, the Nobel Price for the CT scan. CT scan is a technique which composes a routine in a hospital’s everyday life in the whole world. Τhe discovery was made by A. Cormack and G. Hounsfield (award in 1979), Electrical Engineers (experiments in pigs). Additionally, the discovery of the use of radiolabel molecules in diagnostic approaches (RIA). The experiments were performed on rabbits by Rosalyn Sussman Yalow, a physicist (award in 1977). This technique is a widespread need in hospitals all over the world and in large diagnostic laboratories (Private and Public). Furthermore, the Nobel Prize in Chemistry is not only given to chemists. This is also given to scientists with a very important contribution in the scientific field. For example, Dr. Aaron Ciechanover in 2004, for his research on proteins and applications in hematological diseases. So it is not only given to chemists but also to other scientists such as doctors. All the above give us three significant messages: – The interdisciplinary approach and cooperation of scientists and their specialties is the key to significant results. – Experimental research using laboratory animals touches almost all scientific fields. – Even in our time of great technological explosion and evolution, the use of animal models remains a necessity for research and the development of new products and therapies
NASCE (Network of Accredited Clinical Skills Centres of Europe) is a section of the European Union of Medical Specialists (UEMS). The largest scientific body in Europe with almost 2 million MDs. It also has strong links and relations with European Institutions (Commission and Parliament), the other independent European Medical Organizations and the European Medical / Scientific Societies.
NASCE : 1 -Certify highest standards of education (patient safety), 2 -Advance science of clinical education, training and assessment 3 – Enhance standards for operation of Clinical Skills Centres through assessment and accreditation.
Also, NASCE accredits centres with different specialties, increase collaboration network and share expertise with other accredited NASCE centres. Furthermore, promotes joint Research Opportunities, organize Train the Trainer and Train the Simulation Technician Courses and of course increase significantly international recognition.

Apostolos E. Papalois, graduated from the Department of Biology University of Athens in 1991. His PhD Thesis was elaborated in the University of Athens (Department of Biology and School of Medicine) : Isolation and transplantation of pancreatic islets and hepatocytes and bioartificial liver. His post graduate and PhD training contains University of Minnesota, University of Cambridge, University of Boston, University of California Los Angeles and also special training courses (Transplantation, Laboratory Animal Science. Courses, Leadership – Harvard). In 1996 he was appointed Director of the Experimental, Educational and Research Center ELPEN Pharmaceuticals (24 years and 8 months – until December 2020).
Teaching duties in pre – and post – graduate level in Universities of Athens, Thessaloniki, West Attica, Alexandroupolis -Thrace and Patras, after official academic appointment. Visiting Professor (University of Ioannina, Greece, Timisoara, Romania, San Antonio, Texas and Harvard Medical School three times). Today is DeputyDirector of two Experimental Labs in the University of Athens, Secretary General of NASCE / UEMS Executive Board, President of the Scientific Council of Special Unit for Biomedical Research and Education of the Medical School in the Aristotle University of Thessaloniki, Member of the National Committee for Experimental Research and Member of the Scientific Council for HR and Skills of the National Committee of Research, Technology and Innovation. Also Director of Translational Research and Education in HEAL Academy (Hellenic Healthcare Group). Totally 348 publication in Pub Med (peer review journals) and award and distinctions from national and international scientific societies and bodies.
- 11:00 – 12:00 Coffee break
- 12:00 – 13:30 Scientific Session II: Pharmacological targeting of NRF2 (II) Chair: Santiago Cuevas
Pharmacology of Electrophilic NRF2 Activators
Albena T. Dinkova-Kostova
Division of Cellular and Systems Medicine, University of Dundee School of Medicine, United Kingdom
NRF2 and its principal negative regulator, KEAP1, control the expression of genes encoding cytoprotective proteins that provide adaptation to oxidative, electrophilic, inflammatory,and metabolic stress. Pharmacologic NRF2 activation is protective in numerous models of human disease, and NRF2 is an attractive therapeutic target, particularly for chronic conditions, where oxidative stress and inflammation underlie disease pathogenesis. Several NRF2 activators are currently in various stages of drug development, and two electrophiles, the fumaric acid ester dimethyl fumarate and the cyanoenone omaveloxolone, are already in clinical practice. Electrophiles modify sensor cysteines of KEAP1, preventing NRF2 ubiquitination and free KEAP1 regeneration, thereby allowing accumulation of de novo synthesized NRF2. KEAP1 is a flexible sensor, and although cysteine 151 is the primary sensor for electrophiles, such as sulforaphane and the cyanoenone NRF2 activators, at high concentrations, other cysteines (in KEAP1 and other cellular proteins) can be modified. Unlike many other therapeutic agents, the consequences of pharmacologic NRF2 activation are long-lasting and exceed the half-life of the drug, allowing efficacy with intermittent administration. Human intervention studies have shown that electrophilic NRF2 activators, such as sulforaphane, are absorbed rapidly and metabolized via the mercapturic acid pathway, with dose-dependent excretion profiles. A biomarker exploration study assessing the mRNA levels of a panel of classical NRF2 targets and pro-inflammatory cytokines shows promise for monitoring pharmacodynamic responses and guiding biomedical interventions. Many challenges remain, including target specificity, monitoring target engagement, short/long-term safety considerations, identifying the most appropriate disease indications, and understanding the extent and implications of variation in NRF2 activity.

Albena Dinkova-Kostova is a Professor of Chemical Biology at the University of Dundee School of Medicine (UK). She graduated in Biochemistry and Microbiology from Sofia University (Bulgaria) and obtained her PhD degree in Biochemistry and Biophysics from Washington State University (USA). She subsequently trained in Pharmacology at Johns Hopkins University School of Medicine (USA), where she continues to hold an Adjunct Professor position. She joined the University of Dundee in 2007 as a Research Councils UK. Academic Fellow and a research group leader. Her group collaborates with basic scientists and clinicians, and with the pharmaceutical industry. In her research, at the interface of Chemical Biology and Medicine, she is committed to understanding how cells and organisms respond to oxidative, inflammatory, and metabolic stress, and is working towards development of strategies for protection against chronic disease. She was named among the top influential academics in Clarivate’s Highly Cited Researchers 2019, 2020, 2021, 2022, and 2023 lists.
Non electrophilic PPI inhibitors
Anders Bach
The production and elimination of reactive oxygen species (ROS) are tightly regulated to prevent damaging oxidative stress and maintain redox homeostasis. However, in many diseases, ROS play a central role, for example by inducing inflammation or cellular degeneration. To generate new chemical probes and drug leads we target protein-protein interactions involved in oxidative stress by using fragment-based drug discovery (FBDD). Our main targets are the adaptor protein Keap1, which serves as an oxidative stress sensor and regulates the endogenous antioxidant response, and the superoxide-generating multi-subunit enzyme complex NADPH oxidase 2 (NOX2). In this talk, I will present the development of our FBDD platform and show recent results related to Keap1.

Anders Bach received his PhD in medicinal chemistry from University of Copenhagen (UCPH) in 2009. Here, he developed compounds against PDZ domains involved in protein-protein interactions (PPIs) in the CNS. A main discovery was the dimeric peptidomimetic PSD-95 inhibitors that resulted in the spinout company Avilex Pharma and recent tests in clinical trials. After a postdoc period at UCPH, he moved to the Italian Institute of Technology in Genoa in 2012, where he worked on covalent small-molecule inhibitors of enzymes involved in lipid metabolism and signalling. In 2016, Anders started his own research group at the Department of Drug Design and Pharmacology (UCPH) with the aim of making biological active small-molecule inhibitors of PPIs involved in oxidative stress and inflammation by using fragment-based drug discovery (FBDD). This has led to several key discoveries against drug targets like Keap1 and NOX2. While this work continues, focusing on various lead optimization strategies, his FBDD platform is being applied to new protein targets of relevance to therapeutic areas such as CNS diseases, inflammation, and cancer. Anders was recently appointed professor and head of studies of the Master of Science programme in medicinal chemistry, and he teaches in and runs courses related to drug discovery and development.
Development of NRF2 activators for the treatment of dermatological diseases.
Nissim L., Saraç BE., Kahremany S., Ogen-Shtern N., Cohen G., Karaaslan C., Gruzman A.*

Arie Gruzman
Associate Professor, Department of Chemistry, Bar-Ilan University, Ramat-Gan, Israel.
The Field Editor (Medicinal Chemistry) of “Pharmacological Reports”.
National Representative of the Chemistry and Human Health Division of World Chemical Organization (IUPAC).
- 13:30 – 15:30 Lunch break
- 15:30 – 17:00 Scientific Session III: Models and tools to study NRF2 pharmacology (I) Chair: Albena Dinkova-Kostova
In vivo experimental platforms to study NRF2
Ioannis P. Trougakos
Professor, Faculty of Biology, National and Kapodistrian University of Athens, Athens, 15784, Greece.
Email: itrougakos@biol.uoa.gr
Web: http://scholar.uoa.gr/itrougakos/home
A central module of antioxidant defenses in higher metazoans refers to the ubiquitously expressed NFE2-related transcription factor 2 (NRF2), which along with its redox-sensitive cytoplasmic inhibitor Kelch-like ECH-associated protein 1 (KEAP1), defends tissues against unbalanced oxidative load, providing thus protection against oxidative damage of biomolecules. The NRF2-KEAP1 system is seemingly evolutionarily conserved in different organisms of the animal kingdom and studies in various model organisms (e.g., mice, zebrafish, Drosophila melanogaster and Caenorhabditis elegans), including recent advances in genome projects, have provided important information regarding the evolvement of these anti-stress machineries during evolution. We will discuss the NRF2-KEAP1 system in model animals, providing also input on the evolutionary history of this ancient cell defense system.

Ioannis Trougakos obtained his Ph.D. in Cellular-Developmental Biology from the National and Kapodistrian University of Athens (NKUA), Greece. He has worked as Research Scientist at EMBL, Germany, CBM “Severo Ochoa”, Spain and at NHRF, Athens, Greece; he was also research visitor at EMBL and at the Netherlands Cancer Institute. Dr. Trougakos was elected Research Lecturer at NHRF and currently he serves as Professor and Director of the “Cell Biology” lab at the Faculty of Biology, NKUA. He is the Head of the “Ageing and Age-Related Diseases” group (http://scholar.uoa.gr/itrougakos). Dr. Trougakos has published articles in high-ranking journals, chapters in international books; he is also co-inventor in several patents. His group is funded by private (GR, EU, USA) and public (GR, EU) entities; also, the group participates in contractual activities with the Industry.
Mouse models of neurodegenerative diseases: Alzheimer and Parkinson
Ana I Rojo
Department of Biochemistry and Instituto de Investigaciones Biomédicas Alberto Sols UAM-CSIC, Faculty of Medicine, Autonomous University of Madrid, Madrid, Spain. Centro de Investigación Biomédica en Red Sobre Enfermedades Neurodegenerativas (CIBERNED), Instituto de Investigación Sanitaria La Paz (IdiPaz). airojo@iib.uam.es
Alzheimer’s and Parkinson’s diseases (AD and PD, respectively) are most commonly characterized by loss of homeostasis during aging or result from environmental factors, which lead to low-grade sustained generation of reactive oxygen species (ROS), chronic inflammation, and metabolic imbalance. Nuclear factor erythroid 2 p45-related factor 2 (NRF2) is a basic leucine-zipper transcription factor that regulates the cellular redox homeostasis. NRF2 controls the expression of a battery of more than 250 human genes that share in their regulatory regions a cis-acting enhancer termed the antioxidant response element (ARE). The products of these genes participate in a broadly cytoprotective program comprising biotransformation and redox reaction, lipid and iron metabolism, inflammation, proteostasis, and mitochondrial bioenergetics. Research on mouse models has provided tremendous knowledge of the molecular mechanisms by which NRF2 affects AD and PD pathogenesis. We discuss the validity and use of these models and identify future challenges. In conclusion, NRF2 emerged as a crucial modulator of brain homeostasis, providing a new avenue for exploring its potential as a therapeutic target for neurodegenerative diseases characterized by progressive neuronal loss, such as PD and AD

Ana I Rojo. Ana I Rojo studied Biochemistry and Molecular Biology at the Autonomous University of Madrid (2001 and 2002), holds a PhD in Biochemistry (graduated in 2006), and since 2017 is professor in Biochemistry at the Autonomous University of Madrid (Faculty of Medicine). As professor, she has participated in multiple teaching activities for the degrees of Biochemistry, Medicine, and Nursing, with special focus on research training. She has been holder of different competitive fellowships and contracts. Her professional career is focused on the study of the molecular basis of neurodegenerative diseases and in the search for novel brain protective therapies with a special focus on redox biology and NRF2 transcription factor. Nowadays, she is exploring the role of NRF2 in the pathogenesis of Alzheimer’s disease and lateral amyotrophic sclerosis as principal investigator. She has published over 50 primary and review articles and participated in more than 30 congress.
Genetic models of Nrf2 in thyroid
Gerasimos Sykiotis
Senior Staff Physician and Associate Professor
Lausanne University Hospital and University of Lausanne. gerasimos.sykiotis@chuv.ch
My group studies the roles of Keap1/Nrf2 signaling in the thyroid for over 10 years. We use mice (global and thyroid-specific Nrf2 knockout mice, and global Keap1 hypomorphic mice that have activated Nrf2 signaling), thyroid follicular cells lines and organoids, and patient clinical data and samples (serum, DNA, thyroid). Our initial motivation was that thyroid hormone synthesis requires the active generation of hydrogen peroxide that is used to trigger a series of oxidation reactions leading to the iodination of thyroglobulin, the main protein product of the thyroid and the precursor of thyroid hormones. First, we confirmed that Keap1/Nrf2 signaling protects the thyroid against oxidative stress. Then, we went on to identify numerous additional roles of Nrf2 in thyroid physiology and pathophysiology, including the following: (i) direct and positive regulation of the transcription of the gene encoding thyroglobulin; (ii) indirect negative regulation of the iodination of thyroglobulin protein; (iii) protective role against thyroid autoimmunity (Hashimoto’s thyroiditis, a very common disease); (iv) role in goiter (thyroid enlargement) that is caused in mice and humans by excessive genetic activation of Nrf2 signaling; and (vi) pro-oncogenic role in thyroid cancer. We can contribute to two types of translational research studies on Nrf2: First, studies focusing on Nrf2 as a drug target to treat thyroid diseases (on-target therapeutic effects). Second, studies targeting Nrf2 for non-thyroidal diseases test (on-target side-effects). Regarding the latter, we have already published papers supporting the thyroidal safety of a sulforaphane-containing regimen for chemoprevention, and of dimethyl fumarate in multiple sclerosis.

Gerasimos Sykiotis. I am a physician-scientist specialized in clinical and basic endocrinology with a particular focus on thyroid physiology and thyroid diseases, including thyroid cancer. Since 2015, I am responsible for the thyroid clinic at the Endocrinology Service of the CHUV (Lausanne University Hospital). My basic research, funded primarily by the Swiss National Science Foundation, focuses on the roles of cellular antioxidant response systems in thyroid physiology and pathophysiology. My clinical research focuses on the needs of patients with thyroid diseases, including quality of life among thyroid cancer patients and survivors.
- 17:00 – 17:30 Coffee Break
- 17:30 – 19:00 Scientific Session IV: NRF2 Monitoring, Drug Targets and Delivery Chair: Ángela Valverde
Kidney disease in preclinical models. Drug testing
Juan Antonio Moreno
Senior Staff Physician and Department of Cell Biology, Physiology and Immunology, Cordoba University, Spain
juan.moreno@imibic.org
Renal disease is a common cause of death worldwide. However, therapies to prevent progression of this pathology remains an unmet need. Renal injury is associated to increased oxidative stress, inflammation, fibrosis and cell death. These pathological responses may be regulated by Nrf2 (nuclear factor erythroid 2-related factor 2). In fact, pre-clinical and clinical studies have demonstrated the beneficial effects of Nrf2 activation against renal diseases, in both chronic kidney disease (CKD) and acute kidney injury (AKI). In this presentation we will review current knowledge on the protective mechanisms mediated by Nrf2 against kidney injury and we will discuss the effects of different compounds targeting Nrf2 in renal diseases.

Juan Antonio Moreno is a Ramon y Cajal Tenure Track Researcher at the Department of Cell Biology, Physiology and Immunology (Cordoba University, Spain). Head of the Group GE-06 “Pathophysiology of renal and vascular damage” at Maimonides Research Institute of Cordoba (IMIBIC). Our group is unravelling novel pathogenic mechanisms involved in the development of renal and vascular diseases (atherothrombosis, diabetic nephropathy, glomerular diseases, acute kidney injury, renal fibrosis, among others). We aim to understand the basis of the alterations occurring in both kidney and arterial wall by targeting specific pathways to decrease the progression of these pathologies. Specifically, we are interested in certain cellular and molecular mechanisms, such as oxidation, inflammation, apoptosis and fibrosis. In the last years, we have evaluated the role of Nrf2 in acute kidney injury and are interested in the validation of novel compounds targeting Nrf2 to decrease both renal and vascular damage.
NRF2 role in the regulation of NLRP3 inflammasome activation in diabetic nefropathy
Esther Jumilla, Antonio Perez-Olmos, Celisa Arias-Sanchez, María José Caballero-Herrero, Santiago Cuevas
Senior Staff Physician and BioMedical Research Institute of Murcia (IMIB-FFIS), Murcia (Spain)
Santiago.cuevas@imib.es
The inflammasome is a critical regulator of renal inflammation and a key factor in the pathogenesis of kidney disease. The renal DJ-1 protein has antioxidant and anti-inflammatory properties and is able to prevent Nrf2 degradation. To explore novel pharmacological applications of the Nrf2/DJ-1 pathway, we designed ND-13, a peptide consisting of 13 highly conserved amino acids from the DJ-1 sequence. Mouse bone marrow macrophages (BMM) were treated with bardoxolone ( Nrf2 activator) and ND-13. Diabetes was induced in C57Bl/6 mice by injection of streptozotocin (STZ) and treated with ND-13. Peripheral blood mononuclear cells (PBMC) were isolated from diabetic nephropathy patients and controls, plated, stimulated with LPS/ATP, and treated with ND-13 and bardoxolone. IL-1β concentration in BMM medium increased after stimulation of NLRP3 inflammasome by LPS/ATP, ATP and decreased in macrophages pretreated with bardoxolone but not with ND-13, but in the presence of H2O2 (100 nM) ND-13 significantly decreased IL-1β release. Induction of diabetes in mice by STZ showed increased IL-1β release after cell stimulation with LPS+ATP in peritoneal macrophages, suggesting that the inflammasome may be activated in diabetes, although, treatment with ND-13 normalized its activity. Significant differences were found in the expression of COL-I, COL-II, TGF-β, IL-6, TNF-α, and P2X7, whose increase was partially prevented by ND-13 pre-treatment. PBMC from patients with diabetic nephropathy pretreated with LPS/ATP showed a tendency to increase IL-1β release compared to controls. All these data indicate that inflammasome activation in peripheral immune cells is associated with diabetic nephropathy, suggesting a possible role for ND-13 in diabetic nephropathy. ND-13 may be a new approach to attenuate inflammasome activation and fibrosis in kidney disease.

Santiago Cuevas. Principal Investigator Miguel Servet. Molecular Inflammation Group. BioMedical Research Institute of Murcia (IMIB-FFIS)
22 years of experience in the academy and industry in basic, translational and clinical research studying the pathways involved in the regulation of oxidative stress and inflammation in the pathogenesis of hypertension, cardiovascular and renal diseases. Ten years of research experience in the United States on the field. At the present, I am a Principal Investigator Miguel Servet in the Institute of Biomedical Research in Murcia (IMIB). I am a team leader in the Unit of Molecular Inflammation at the IMIB led by Dr. Pablo Pelegrin, where several groups study the molecular mechanism involved in inflammasome regulation and its role in the pathogenesis of several diseases.
Mitigating the effects of ageing using TXA302 as a senotherapeutic agent
Paul Shiels
Professor of Gerosicence
School of Molecular Biosciences MVLS
University of Glasgow. paul.shiels@glasgow.ac.uk
Biological ageing ( ‘miles on the clock’) is a major driver of a range of age-related diseases, such as chronic kidney disease, cancer, cardiovascular disease, and neurodegeneration. In order to improve health span (years of healthy living), a range of senotherapies are being developed to mitigate the molecular and cellular deficits of the ageing process. We have assessed the potential of repurposing the angiotensin-(1-7) analogue TXA302, a clinically licensed agent known to mitigate inflammatory burden , as a senotherapeutic agent capable of slowing the rate of organismal ageing and improving health span. To do so, we have explored the mechanistic basis of this activity during normative ageing.
A study was conducted comparing treatment (TXA302) vs. control (PBS) in C57BL/6J mice (N=102) of both sexes over an age rage spanning 3-18 month. To assess health span, we measured a range of biophysical, biochemical and cellular markers of ageing, (including grip strength, glucose tolerance, IL-6, Nrf2, gut microbiome composition, epigenetic age, and expression of the CDK inhibitors CDKN1A, CDKN2A, and CDKN2B.
Treatment with TXA302 resulted in significant improvements in health across multiple measures. TXA302-treated mice displayed better age related grip strength (p=0.028), improved glucose tolerance (p=0.004) and lower plasma IL-6 levels (p=0.046). CDKN2A expression, a proven marker of cellular senescence, was 34% lower in the treated group (p=0.044). Physiological improvements also correlated with increased alpha diversity in the gut microbiome index (p=0.001).
Significantly, we observed non change in epigenetic age.
TXA302 was well tolerated and improved numerous parameters subject to age-associated decline. These results indicate that targeting the angiotensin signalling pathway through an angiotensin-1-7 analogue is a promising novel strategy for the purpose of improving health span.

Paul Shiels is Professor of Geroscience at the University of Glasgow. He is a a founder member of the Glasgow Geroscience Group. He has over 200 publications and generated 10 Patents in this field. Paul was a pioneer of telomere cloning and worked on ageing in cloned animals, including Dolly the sheep, at PPL Therapeutics Roslin. He has pioneered the concept of the exposome of ageing and was subsequently the first to describe links between socioeconomic position, the microbiome and ageing. His ideas have been successfully tested in clinical trials. His current research portfolio comprises investigation of the exposome of ageing, novel senotherapies and development of biomarkers of ageing, including epigenetic clocks.
He has recently developed the first clinically accurate epigenetic clock for normative ageing.
Paul holds the honour of being Chair of the Scientific Advisory Board of the British Society for Research on Ageing, the world’s oldest charitable society for research on ageing. He has acted as an expert on the Biology of Ageing on a number of national policy advising consortia including providing evidence to UK XIRA and the UK Government All Party Parliamentary Group on Longevity.He is Chair of the FWO (Belgium) Med 6 Fellowship Funding Panel and is a panel member for the UK Research Partnership Investment Fund (UKRPIF). He sits on the Editorial Advisory Board for Aging Cell, Frontiers in Aging, Current Aging Biology. He sat on the International Advisory Board for the Nobel Symposium 2019 and was a Plenary Presenter at the Nobel Symposium 2019. He was a discussant at the Marabou Symposium (2022) and is an invitee discussant at the 2024 Symposium.
Paul has acted as CSO for Pathfinder Cell Therapy PLC and has sat on the Scientific Advisory Boards of TC Biopharm and 4D Pharma and acts as a consultant for a range of Pharma companies. He has a proven track record in public dissemination of his research, including the provision of expert commentary for the BBC and ABC TV networks and as a Panellist at the Edinburgh International Science Festival and the Edinburgh International Book Festival. Paul is also a Global Celt, part of an internationally based diaspora supporting where possible the Global ambitions, long term vision and ongoing operational development of Celtic FC and the Celtic FC Foundation. Operating from the most senior level in Industry/ Commerce/ Academia/ Public Office and the Arts he supports the leadership team with shared experiences knowledge and mentoring programmes.
Friday, February 9, 2024
- 09:00 – 11:00 Scientific Session V: Models and tools to study NRF2 pharmacology (III) Chair: Antonio Cuadrado
European Innovation Council in the forefront of innovation in Europe.
About the European Innovation Council
Iordanis Arzimanoglou
Programme Manager
European Innovation Council and SMEs
Executive Agency (EISMEA)
Established by the European Commission
Ioannis Amarantos
Project Adviser
European Innovation Council and SMEs
Executive Agency (EISMEA)
Established by the European Commission
The EIC is Europe’s flagship innovation programme to identify, develop and scale up breakthrough technologies and game changing innovations. The European Innovation Council (EIC) has been established under the EU Horizon Europe programme. It has a budget of €10.1 billion to support game changing innovations throughout the lifecycle from early-stage research to proof of concept, technology transfer, and the financing and scale up of start-ups and SMEs.
The strategy and implementation of the EIC is steered by the EIC Board, which has independent members appointed from the world of innovation (entrepreneurs, researchers, investors, corporates and others from the innovation ecosystem).
The EIC and SME Executive Agency responsible for supporting the EIC Board and its President, and for implementing the EIC’s activities which are set out in an annual EIC Work Programme.
Find out about EIC funding opportunities
The EIC takes a pro-active approach to managing funding under the leadership of EIC Programme Managers who develop visions for innovation and technology breakthroughs and steer portfolios of projects to achieve these goals. EIC Programme Managers have a high level of expertise in their field and are recruited for limited time periods (up to 4 years) to design and oversee EIC funding in their portfolio areas.
A unique feature of the EIC is that it provides funding for individual companies (mainly startups and SMEs) through both grants and investments. The investments currently take the form of direct equity or quasi-equity investments and are managed by the EIC Fund.

Iordanis Arzimanoglou EIC Program Manager for health and biotechnology. Iordanis Arzimanoglou has a thirty-year postdoctoral international career. Since June 2020, he has been the Programme Manager for Health and Biotechnology at the EIC, where he´s responsible for developing visions for technological and innovation breakthroughs. Iordanis has previously worked as an EIC Coach & Senior advisor to genomic/genetic SMEs. Iordanis was the Chief Executive Officer of the Thessaloniki Innovation Zone managing company, and prior to that, Chief Executive Officer of the Aarhus Botech Cluster Company (BMF) and was Associate Professor at the Department of Biomedicine Aarhus University.
Earlier in his career, he was the Assistant Professor of Molecular Genetics at Weill Medical College of Cornell University, New York and prior to that, he was Chief of Cancer Genetics Research Program at Lennox Hill Hospital, New York. Before joining Lenox Hill, he was a research scientist in the Division of Medical Genetics at Weill Medical College of Cornell University.
Iordanis holds a PhD from the University of Athens, Greece in Molecular Genetics and Biochemistry, a Boston University Graduate Certificate in international business management and performed his postdoctoral work in the Department of Medicine at Weill Medical College of Cornell University, New York.

Ioannis Amarantos Ioannis Amarantos has a twenty-year postdoctoral international career. Since Sep 2020, he has been working for the European Commission managing grants in Health and Biotechnology and working close with the Programme Manager, Iordanis Arzimanoglou at the European Innovation Council (EIC). Ioannis has previously worked for 14 years in Germany as field application scientist, Project Manager, Director of Quality, & Regulatory affairs in biotech startups after his Post-Doctoral at European Molecular Biology Laboratory (EMBL) in Heidelberg.
Ioannis holds a Ph. D in Biochemistry and a MSc. in “Basic Medical Sciences” from the Faculty of Medicine of University of Patras, Greece. He has aquired his BSc. in Chemistry from the Dept of Chemistry of University of Patras, Greece. Ioannis is a cerified Project Manager PMP® from Project Management Institute®, world’s leading authority on project management.
Effect of an intraperitoneal treatment with the antipsychotic olanzapine on the inter-organ crosstalk regulating energy balance and liver metabolism in mice.
Ángela M Valverde.
Instituto de Investigaciones Biomédicas “Sols-Moreale” CSIC-UAM, Madrid, Spain; Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERdem), ISCIII, Madrid, Spain.
Olanzapine (OLA), a widely used second-generation antipsychotic (SGA), causes weight gain and metabolic alterations when administered orally to patients. Recently, we demonstrated that, contrarily to the oral treatment which induces weight gain, OLA administered via intraperitoneal (i.p.) in male mice resulted in body weight loss. This protection was due to an increase in energy expenditure (EE) through a mechanism involving the modulation of hypothalamic AMPK activation by higher OLA levels reaching this brain region compared to those of the oral treatment. Since clinical studies have shown hepatic steatosis upon chronic treatment with OLA, herein we further investigated the role of the hypothalamus-liver interactome upon OLA administration in wild-type (WT) and protein tyrosine phosphatase 1B knockout (PTP1B-KO) mice, a preclinical model protected against metabolic syndrome. WT and PTP1B-KO male mice were fed an OLA-supplemented diet or treated via i.p. Mechanistically, we found that OLA i.p. treatment induces mild oxidative stress and inflammation in the hypothalamus in a JNK1-independent and dependent manner, respectively, without features of cell dead. Hypothalamic JNK activation up-regulated lipogenic gene expression in the liver though the vagus nerve. This effect concurred with an unexpected metabolic rewiring in the liver in which ATP depletion resulted in increased AMPK/ACC phosphorylation. This starvation-like signature prevented steatosis. By contrast, intrahepatic lipid accumulation was observed in WT mice treated orally with OLA; this effect being absent in PTP1B-KO mice. We also demonstrated an additional benefit of PTP1B inhibition against hypothalamic JNK activation, oxidative stress and inflammation induced by chronic OLA i.p. treatment, thereby preventing hepatic lipogenesis. The protection conferred by PTP1B deficiency against hepatic steatosis in the oral OLA treatment or against oxidative stress and neuroinflammation in the i.p. treatment strongly suggests that targeting PTP1B might be also a therapeutic strategy to prevent metabolic comorbidities in patients under OLA treatment in a personalized manner.

Ángela M Valverde. nstituto de Investigaciones Biomédicas Alberto Sols (CSIC-UAM) Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERdem)
My research career has been focused in molecular metabolism. As PhD fellow (1988-91), I studied insulin/IGF1 actions in brown adipocytes. As postdoc at Cancer Research UK (London, 1991-93), I cloned protein kinase D1, a Ser/Thr kinase that later studies revealed its fundamental role in cancer and recently, in glucose homeostasis. Back to Spain as Assistant Professor at Complutense University of Madrid, I implemented my expertise in signal transduction in unraveling insulin/IGF1 cascades related to proliferation/differentiation of brown adipocytes. From 2002 and as a Senior Scientist at Spanish National Research Council (CSIC), I explored mechanisms of insulin action in the liver, particularly IRS2 and PTP1B as opposite modulators of insulin sensitivity. In 2006, I moved to the Institute of Biomedicine Alberto Sols (CSIC/UAM) where I consolidated my leadership as PI and extended my research to the molecular basis of non-alcoholic fatty liver disease (NAFLD). From 2008, I am Principal Investigator at CIBERdem (Spanish network in research on diabetes and related metabolic diseases). In this topic, we studied the impact of insulin resistance, ER stress, autophagy, oxidative stress and inflammation in NAFLD. A step further, we investigate the interactome between liver cells in the NAFLD context. We are currently extending this interactome to the extracellular vesicles field and hepatic progenitor cells. Regarding NAFLD therapeutics, we unraveled the efficacy of a GLP1/Glucagon receptor co-agonist in improving liver regeneration during NASH. In the last 10 years, our lab has also studied the molecular basis of diabetic complications, i.e. retinopathy and nephropathy, as well as drug-induced hepatotoxicity. In the context of metabolic alterations induced by chronic drug treatments, we evaluate how antipsychotic medication impacts on whole body metabolism.
Thiosulfate Sulfurtransferase and NRF2 Function in the Brain
Harry van Goor
Thiosulfate sulfurtransferase (TST, EC 2.8.1.1) was discovered as a Rhodenase enzyme that detoxifies cyanide. Its characteristics relate to sulfide metabolism, antioxidant defense and mitochondrial function, which are important protective biological processes during oxidative distress. TST has been described to play an important role in liver and colon tissue. Thiosulfate, a sulfane sulfur and substrate for TST, is thought to activate the NRF2 pathway through release of sulfide, a known activator of NRF2. We studied the effect of TST deficiency in mice on the redox balance in the cerebral cortex, the area of extensive neuronal activities and thereby sensitive to oxidative distress.C57BL/6J and Tst -/- 6-month old mice were used. Cortices were evaluated for antioxidant enzymes protein expression and activity levels via immunoblot and activity assays. The OXPHOS proteins and mitochondrial respiration were measured by immunoblot and high-resolution respirometry. Fluorescent probes were utilized to measure ROS and RSS. Total ATP level was detected by luminescence assay. Tst -/- mice had a striking 20-fold and 475-fold elevation in the trunk plasma and urine of thiosulfate compared to WT. The cortex of the Tst -/- mice contains significantly decreased level of H 2 S and increased H 2 S n . We found a dysregulated pathway downstream of H 2 S, as evidenced by decreased GSH/GSSG ratio and GPX4 protein expression. We also observed an increase in total ATP level in Tst -/- cortices. High-resolution respirometry showed that Tst -/- cortices have an increase in mitochondrial complex IV activity, suggesting mitochondrial fitness. H2O2 and O 2- , as products of OXPHOS, were found increased in Tst -/- cortical brain samples. Finally, we found a significant decrease in SOD activity and an increase in catalase activity in Tst -/- cortexes compared with WT. Nrf2 protein and Keap1 protein displayed disrupted expression levels in Tst -/- mice brain samples compared to WT. We provide a first glimpse into the molecular and metabolic changes of TST deficiency in the brain and suggest that pathophysiological conditions associated with aberrant TST expression and/or activity renders neurons more susceptible to oxidative stress-related malfunction via NRF2/Keap1 associated mechanisms. We now developed and patented an oral thiosulfate formulation which can serve as a substrate for TST thereby inducing benefical effects on a.o. the NRF2 pathway. This has already been proven in models for liver disease. The tablet is acid resistant and releases thiosulfate in a controlled fashion. As has special feature the tablet retains its slow release features even after being damaged. An SME is currently being set-up to further develop the application of thiosulfate in human disease.

Harry van Goor is a fundamental researcher and a translational scientist at the University Medical Center Groningen, the Netherlands. He completed his PhD program at the University of Groningen and a post-doctoral fellowship at the Penn State University, Hershey, PA, USA. He became full professor in 2010 at the University of Groningen, the Netherlands. He is currently involved in various projects focusing on the role of the reactive species interactome i.e. ROS, NO and H2S in COVID-19, aging, neurodegeneration, cardiovascular disease and the metabolic syndrome. In recent years he started studying the role of NRF2-KEAp1 signaling in relation to reactive species stress in various conditions. Since sulfane sulfurs, such as thiosulfate, are thought to activate Nrf2 signaling pathways through the structural change of Keap1 protein and phosphorylation of AKT, he has now patented an oral formula for thiosulfate, which may pave the way for therapeutic interventions. An SME will soon be set up to further develop this product for human applications.He has published over 400 scientific papers in peer reviewed journals and has a citation index of almost 100. His current research group consists of 10 MD/PhD students and PhD students from different nationalities and backgrounds. Forty PhD students already acquired their PhD under his supervision in recent years. Harry van Goor has received several research grants from the Dutch Kidney Foundation. He has served for three years as President of the International society of Antioxidants, Nutrition and Health with yearly meetings in Paris, France. He has been a member of the organizing committee for 4 years of the World Congress on Hydrogen Sulfide in Biology and Medicine. He currently serves as Management team member of the Cost Action on “Bench to bedside transition for pharmacological regulation of NRF2 in non-communicable diseases (BenBedPhar)" representing the Netherlands
Therapeutic Application of Patient-Derived Liver Organoids in Metabolic Dysfunction-Associated Steatohepatitis and Liver Fibrosis.
Bruno Ramos-Molina.
Biomedical Research Institute of Murcia (IMIB), Murcia, Spain.
Metabolic dysfunction-associated steatohepatitis (MASH), characterized by inflammation, hepatocellular ballooning, and hepatic steatosis, affects 2-3% of the adult population, with higher prevalence among individuals with obesity and type 2 diabetes. MASH is not only associated with the development of severe hepatic conditions like advanced fibrosis, cirrhosis, and hepatocarcinoma, but it also contributes to increased mortality, primarily from cardiovascular events. Despite its relatively high incidence and potential detrimental effects on human health, effective pharmacological therapies for this chronic liver condition are still lacking.
NRF2, a master regulator of the cellular adaptive response to oxidative stress, has been reported to play a protective role in the gene regulatory program of the antioxidant response against liver diseases, including MASH, in animal models. However, the potential therapeutic use of NRF2 modulation in human MASH models is not well understood. In recent years, new experimental models of liver diseases derived from patient samples have been developed as an alternative to the use of experimental animals. One such alternative is the use of organoids derived from bipotential precursors of the adult liver bile duct. These organoids are genetically stable, amenable to both genetic manipulation and clonal expansion. Recent reports indicate that viable ductal organoids can be directly isolated from liver tissue samples from patients with MASH. These organoids are expandable in the long term, easily undergo hepatic differentiation, and can functionally recapitulate important aspects of the pathology. This model offers a promising avenue to test new therapeutic options for MASH and liver fibrosis, including modulators of NRF2 activity.

Bruno Ramos-Molina is Miguel Servet Researcher and Head of the Obesity, Diabetes and Metabolism Group based at the Biomedical Research Institute of Murcia (IMIB). He achieved his bachelor’s degree in Biochemistry from the University of Murcia (UMU) in 2007, a Master of Biomedicine at the UMU in 2008 and a PhD in Biochemistry at UMU in 2013. After his PhD studies, he performed two consecutive postdocs stages at the University of Maryland (USA) and at the KU Leuven (Belgium). In 2017 he was awarded a Sara Borrell fellowship to join the Biomedical Research Institute of Malaga (IBIMA). During this period at IBIMA he was awarded a research project from the Andalusian Health System as PI. In 2020 he was awarded a Miguel Servet grant by the Health Institute “Carlos III” (ISCIII) to establish his own research group at IMIB. His group has been further consolidated with two national research grants from ISCIII (FIS project numbers PI20/00505 & PI23/00171) and several regional funds. Dr. Ramos-Molina is the author of more than 80 publications in international scientific journals, many of them of great relevance as they are included in Q1 and D1 of their category, including highly prestigious journals in the area of Endocrinology, Metabolism and Nutrition such as Trends in Endocrinology and Metabolism, Diabetes, Metabolism (Clinical & Experimental), Obesity (Silver Spring), Endocrine Reviews, Journal of Clinical Endocrinology & Metabolism, American Journal of Clinical Nutrition, JHEP
Reports, among others. In addition, he is a frequent reviewer for important journals such as Metabolism (Clinical & Experimental), Nature Communications, Obesity Reviews, Gut Microbes, Microbiome, among many others, and is Associate Editor of the journals Diabetes & Metabolic Syndrome: Clinical Research & Reviews and Frontiers in Endocrinology. In turn, he is an active evaluator for the State Research Agency (AEI) and the ISCIII, and for other regional agencies. He has also been an evaluator for important international research agencies such as the European Research Council (ERC) or the Agence Nationale de la Recherche (ANR, France), among others. He has been an invited speaker at important national conferences such as those organized by the Spanish Society for the Study of Obesity (SEEDO) and the Spanish Society for Biochemistry and Molecular Biology (SEBBM) and selected as a speaker at international conferences organized by the Gordon Research Conferences. (GRC) and the European Molecular Biology Organization (EMBO). Recently, he has been session chair and invited speaker of the 6th Global NASH Congress held in London (UK). The applicant also has experience in the organization of scientific events, as he co-organized the VII and VIII IMIB conferences held in Murcia the last two years. Finally, the applicant has extensive international experience and actively collaborates with different international groups of excellence from Europe, Asia, and North America. In addition, he is currently a participating member of the COST EpiLipidNET (CA19105) and BenBedPhar (CA20121) actions and is a member of the advisory board of the “International Polyamines Foundation” since 2019.
- 11:00 - 12:00 Coffee Break
- 12:00 – 13:30 Scientific Session VI: Progress towards the clinic (I) Chair: Harry Van Goor
Activation of the GABA-A receptor counteracts hepatic steatosis and fibrosis: A novel first-in-class approach for MASH therapy
Juergen Eckel
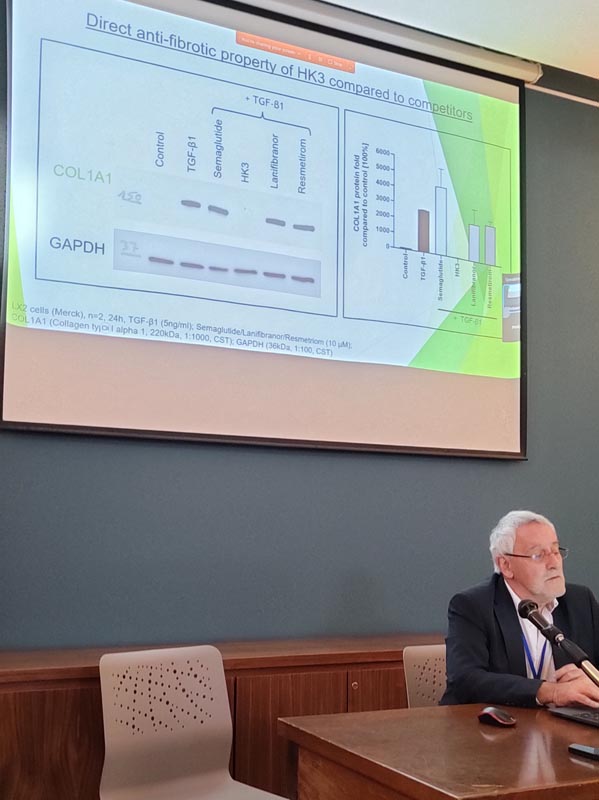
CureDiab has identified thioacrylamide-derivatives (HK1,3,4) that mediate activation of peripheral GABA-A receptors, resulting in hepatoprotection, reduction of steatosis and an anti-fibrotic response. This was demonstrated by reduced caspase 3/7 activity in a lipotoxic model of human hepatocytes involving inhibition of STAT3 and NF-kappaB phosphorylation. In activated LX2 stellate cells, treatment with HK1,3 strongly prevented the upregulation of the profibrotic markers collagen, fibronectin and alpha-smooth muscle actin. Transcriptomic analysis confirmed that thioacrylamides counter-regulate the expression of a pro-fibrotic gene signature. In a mouse model of liver fibrosis HK3 was found to reduce the hepatic fibrosis score and the liver collagen content. In a unique human spheroid MASH model, the anti-steatotic, anti-inflammatory and anti-fibrotic effect of HK3 was confirmed. Overall, HK3 represents a novel approach for MASH therapy.

Juergen Eckel is a Professor of Clinical Biochemistry and he has been working at the German Diabetes Center in Düsseldorf since 1978. His field of study includes insulin signaling, insulin resistance, type 2 diabetes, obesity, adipose tissue and skeletal muscle biology, and organ crosstalk. His research group was the first to directly demonstrate the communication between human adipocytes and skeletal muscle cells. From 2006 to 2011, Prof. Eckel worked as the Acting Director of the Institute of Clinical Biochemistry and Pathobiochemistry at the German Diabetes Center. From 2011 to 2016 he directed the Paul-Langerhans-Group for Integrative Physiology, from 2019 to 2022 he was Director of a Center of Competence at the German Diabetes Center. Since 2022 he is co-founder and CEO of CureDiab Metabolic Research GmbH. Prof. Eckel served as a chairperson for several EU COST Actions between 2000 and 2011 and coordinated the EU FP7 project ADAPT. He received several awards and published more than 200 original papers and reviews.
Targeting NRF2 in NASH
Antonio Cuadrado
SERVATRIX Biomed S.L. is a Spin-off of the Autonomous University of Madrid (UAM), Spain, (https://servatrix.eu/.) and aims to provide an innovative strategy for the treatment of a highly prevalent disease worldwide: non-alcoholic steatohepatitis (NASH) and its progression towards liver fibrosis and cirrhosis. SERVATRIX business plan is to carry out the preclinical development and the first clinical phases of the new target and small molecule identified by Prof. Antonio Cuadrado (UAM), as a novel mechanism for activation of the transcription factor NRF2, essential in protection against oxidative, inflammatory and metabolic stress. These are three fundamental pathological hallmarks of this liver disease. This molecule is a disrupter of the interaction between the E3 ligase adapter beta-TrCP and NRF2. Both the target and the small molecules have been patent protected. In this communication, we will present safety and efficacy data in preclinical models of NASH.

Antonio Cuadrado is a full professor of Biochemistry and Molecular Biology at the Department of Biochemistry, Medical School, Autonomous University of Madrid. He is also co-founder of the spin-off SERVATRIX Biomed S.L. He obtained his PhD degree in Biology in 1985 and enjoyed several postdoctoral stays in the National Cancer Institute-NIH with the help of Fulbright and Fogarty fellowships. He established his independent laboratory as Professor of Biochemistry in 1997 with a main interest on the study of molecular mechanisms involved in initiation and progression of chronic diseases. For the past years his main lane of research has been the validation of transcription factor NRF2, master regulator of cellular homeostasis as a new therapeutic target in NASH. His current interest is the development of new NRF2-modulating drugs. Dr. Cuadrado has published over 160 primary and review articles, of which more than 80 are related to the role of NRF2 in physiological and pathological responses to disease.
Hexaraphane (6-MSITC) as a nutraceutical for NRF2 activation and protector against tauopathy
Ángel Juan García-Yagüe
Instituto de Investigaciones Biomédicas “Sols-Morreale” UAM-CSIC,
Departament of Biochemistry, School of Medicine, Autonomus University of Madrid, Spain. ajgarcia@iib.uam.es
A hallmark of Alzheimer’s disease (AD) is the production of amyloid and TAU alterations that may participate or be the cause of brain metabolic changes, overproduction of reactive oxygen species, and uncontrolled neuroinflammation, leading to neuronal death. The transcription factor NRF2, a master regulator of homeostatic responses, emerged as a new target to combat metabolic, oxidative, and inflammatory stress as well as proteinopathy, all of which are well-characterized symptoms that participate in the onset and progression of AD. TAU is abnormally hyperphosphorylated and is accumulated as intraneuronal tangles of paired helical filaments (PHF), twisted ribbons, and or straight filaments. NRF2 may be up-regulated pharmacologically by several classes of electrophiles including isothiocyanates such as Sulforaphane (SFN) or Hexaraphane (6-MSITC). The mechanism of action of isothiocyanates is the inhibition of the KEAP1, a classical NRF2 repressor. Due to the great relevance of the hyperphosphorylation of TAU (p-TAU) protein in disease, this work intends to study whether the 6-MSITC can regulate the levels of p-TAU and know which action mechanism it. Preliminary data obtained in nerve hippocampus cell lines have revealed that 6-MSITC can decrease the specific toxic levels of p-TAU epitope PHF1, belonging to phosphorylation in pSer396 and pSer404. Hence, we show that 6-MSITC regulates p-TAU levels through an independent mechanism of NRF2 action. These mechanisms also are independent through signaling pathways that regulate GSK-3β activity. Cytotoxic forms of p-TAU are usually recycled through the autophagy process. In this context, we observed that blocking autophagy flux with specific inhibitors and subsequent treatment with 6-MSITC impair p-TAU PHF1 degradation. Moreover, we detected that 6-MSITC can inhibit between 23%-25% GSK-3β activity “in vitro” assay.

Ángel Juan García-Yagüe studied Biochemistry at the Autonomous University of Madrid (2006), holds a PhD in Biochemistry (2012), and since 2012 has been hired as a scientific researcher in the same university under the supervisor of the Cuadrado lab. During his professional career, he had focused at to study and understanding the neurological process that drives neurodegenerative pathology disease, on focus Parkinson’s disease, through the physiopathological mechanics study of the transcription factor NURR1. Currently, he is leading several research projects to focus on the NRF2 role in brain physiology and pathology related to blood-brain-barrier integrity, search for new NRF2 inductors, and collaborating with the Kinjirushi Wasabi Ltd company for research about novel pharmacological approaches in preclinical models of neurodegenerative diseases.
- 13:30 – 15:30 Lunch break
- 15:30 – 17:00 Scientific Session VII: Progress towards the clinic (II) Chair: Gerasimos Sykiotis
Targeting NRF2-KEAP1 for the treatment of Metabolic Associated Steatotic Liver Disease.
Philippe Delerive,
SERVIER, FRANCE (currently employee at Nestle Health Science, Lausanne, Switzerland – philippe.delerive@nestle.com)
Oxidative stress is recognized as a major driver of non-alcoholic steatohepatitis (NASH) progression. The transcription factor NRF2 and its negative regulator KEAP1 are master regulators of redox, metabolic and protein homeostasis, as well as detoxification, and thus appear to be attractive targets for the treatment of NASH. Using fragment screening and structure-based drug discovery, we identified S217879, as a novel and selective molecule able to disrupt the KEAP1-NRF2 interaction. Molecular and cell-based assays confirmed that S217879 is a highly potent and selective NRF2 activator with both antioxidant and anti-inflammatory properties. In methionine and choline-deficient diet (MCDD) mice model, S217879 treatment for 2 weeks led to a dose-dependent reduction in NAFLD activity score while significantly increasing liver Nqo1 mRNA levels, a specific NRF2 target engagement biomarker. In DIO NASH mice, S217879 treatment resulted in a significant improvement of established liver injury, with a clear reduction in both NAS and liver fibrosis. RNA-sequencing analyses revealed major alterations in the liver transcriptome in response to S217879, with activation of NRF2-dependent gene transcription and marked inhibition of key signaling pathways that drive disease progression. Finally, we evaluated the therapeutic potential of S217879 in human MAFLD using patient-derived precision cut liver slices (PCLS) and showed that S217879 displays anti-steatotic effects, lowers DNA damage, apoptosis, and inflammation and inhibits fibrogenesis in human PCLS from patients with MAFLD. Taken together, our results highlight the potential of selective disruption of the NRF2-KEAP1 interaction for the treatment of NASH and liver fibrosis.

Philippe Delerive,
Nestle Health Science, Lausanne, Switzerland
E-mail : philippe.delerive@nestle.com
Philippe obtained is PhD in Molecular and Cellular Biology from the University of Lille (France) working on the role of nuclear hormone receptors in atherosclerosis at the Pasteur Institute. After his postdoc at Eli Lilly (USA), Philippe had during the last 20 years different roles in both Pharma and Biotech companies (GSK, Genfit, Galapagos, Servier) from target discovery and validation up to early development with the identification of drug candidates for multiples indications mainly within the cardio-metabolic space. Philippe led Research in Cardiovascular and Metabolic Diseases at Servier, a French Pharma company, where he and his team targeted NRF2-KEAP1 for metabolic diseases such as Type 2 Diabetes and associated complications such as NASH. This work led to the identification of a drug candidate targeting NRF2-KEAP1 interaction for NASH. Today, Philippe is Global Science Lead at Nestle Health Science (Lausanne, Switzerland) where he is supporting through translational approaches the development of novel therapeutic solutions in both cardio-metabolic and rare diseases
DanubeNeuro programs
Vanja Nagy
Neuroscience Program Director
CEBINA, Central European Biotech Incubator and Accelerator
DanubeNeuro, Dedicated CEBINA program
Email: vanja.nagy@cebina.eu
Websites: www.cebina.eu and www.danubeneuro.com
CEBINA, Central European Biotech Incubator and Accelerator, is a company that was founded with the aim of creating, nurturing, and promoting early-stage life sciences companies and academic projects, to develop new medicines and cutting-edge technologies. Through its programs CEBINA scouts for and evaluates innovative drug discovery projects that address critical unmet medical needs with significant commercial potential and strong IP position. Selected projects are matured and accelerated toward de-risked assets developed for out-licensing or new company formation.
Currently, CEBINA is running two independent programs: Danube Labs and DanubeNeuro. Danube Labs is a partnership between CEBINA Bridge Capital and Evotec, a leading drug discovery and development company, that aims to fund 20 projects in a 4 year time period. Of interest for the Danube Labs program are all therapeutic areas, particularly infectious disease and inflammation. Recently launched DanubeNeuro, on the other hand, scouts for projects in the field of neurodegeneration particularly novel therapeutics, diagnostics, biomarkers and imaging platforms. Additionally interesting are innovative solutions for increasing healthspan and resilience in the aging population.
The CEBINA team has decades of experience in entrepreneurship and academic research. In close collaboration with inventors and vetted CRO partners projects are delivered with highest industry standards. By bridging the funding gap in early-stage projects value is created through various exit strategies that provide significant benefit for all stakeholders.

Vanja Nagy obtained her PhD in Basic Biomedical Sciences at the Icahn School of Medicine at Mount Sinai (MSSM), USA, where she elucidated novel molecular pathways supporting synaptic function underlining learning and memory. Following two postdocs, in 2016 she established her independent research group at the Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases, Austria, that focused on uncovering genetic causation and potential therapeutic interventions of neurological disorders. She joined CEBINA as a Neuroscience Program Director in 2023, overseeing the acceleration program DanubeNeuro focused on innovative solutions in the field of aging and neurodegeneration.
Screening natural products for the identification of NRF2 activity modulators
Ioannis P. Trougakos
CEO. GenCell-TSBiotech, Athens, Greece.
Web: https://gencell.gr/
GenCellTS-Biotech specializes in the field of systems biology with a primary focus on combating ageing and age-related diseases. Ageing is a complex phenomenon caused by the time-dependent loss of cellular homeodynamics and consequently of physiological organismal functions. This process is affected by both genetic and environmental (e.g., diet) factors, as well as by their constant interaction. We translate our research at (among others) on-demand screening assays (both cell-free and cell-based, as well as at in vivo experimental models) aiming to (among others) reveal bioactive molecules with anti-ageing and/or anti-age-related diseases (e.g., cancer, neurodegeneration, etc.) activity. The antioxidant response system comprising (among others) the ubiquitously expressed NFE2-related transcription factor 2 (NRF2) and its redox-sensitive cytoplasmic inhibitor Kelch-like ECH-associated protein 1 (KEAP1) defends tissues against oxidative stress, thereby protecting against pathologies that relate to DNA, protein, and/or lipid damage. Therefore, it represents a promising target for the identification of novel biomolecules with a possible anti-ageing and/or anti-age-related diseases activity. Our rational and experimental platforms for identifying modulators of NRF2 activity will be presented.

Ioannis Trougakos obtained his Ph.D. in Cellular-Developmental Biology from the National and Kapodistrian University of Athens (NKUA), Greece. He has worked as Research Scientist at EMBL, Germany, CBM “Severo Ochoa”, Spain and at NHRF, Athens, Greece; he was also research visitor at EMBL and at the Netherlands Cancer Institute. Dr. Trougakos was elected Research Lecturer at NHRF and currently he serves as Professor and Director of the “Cell Biology” lab at the Faculty of Biology, NKUA. He is the Head of the “Ageing and Age-Related Diseases” group (http://scholar.uoa.gr/itrougakos). Dr. Trougakos has published articles in high-ranking journals, chapters in international books; he is also co-inventor in several patents. His group is funded by private (GR, EU, USA) and public (GR, EU) entities; also, the group participates in contractual activities with the Industry.
- 17:00 – 17:30 Coffee Break
- 17:30 – 19:30 Scientific Session VIII: Building bridges. Chair: Paul Shiels
M102, a combined Nrf2 and HSF1 activator for the treatment of Amyotrophic Lateral Sclerosis and other neurogenerative diseases
Raymond. K. Houck
CEO. Aclipse Therapeutics
rhouck@aclipsetherapeutics.com. Tel: +1-412-606-7214
M102 is a disease-modifying drug candidate for treatment of Amyotrophic Lateral Sclerosis (ALS), also known as Motor Neurone Disease (MND) and Lou Gehrig’s disease. M102 activates the nuclear factor erythroid 2-related factor 2 (NRF2) and HSF1 (heat shock factor 1) signaling pathways, which targets multiple genetically validated pathophysiological mechanisms in ALS. M102 has completed its IND-enabling studies and is now moving into a first-in-human trial. M102 is supported by a preclinical efficacy package which includes neuroprotective effects in two ALS-related animal models (SOD1G93A and TDP43Q331K transgenic mouse models) as well as in patient-derived astrocyte/motor neuron co-culture toxicity assays from SOD1, C9orf72 and sporadic ALS patients. With this approach, the development of M102 is aimed at the whole spectrum of ALS patients including familial and sporadic subgroups. Specific genetic markers have been identified that may allow the identification of those ALS patients that will most likely respond to M102 (i.e. M102 responders and non-responders). M102 development is being conducted in collaboration with the Sheffield Institute for Translational Neuroscience (SITraN) at the University of Sheffield, UK.

Raymond K. Houck is the CEO and co-founder of Aclipse Therapeutics and has over 30 years’ experience in drug development. Previously, Mr. Houck was the CEO of Thar Pharmaceuticals, a clinical-stage company developing a treatment for Complex Regional Pain Syndrome, which was acquired by Grunenthal GmbH. Before that, Mr. Houck was the CEO of Automated Cell, a company focused on the discovery and development of antibody therapeutics for the treatment of cancer and before that, he co-founded Suprex Corporation, an analytical instrument and separation science company that was a pioneer in supercritical fluid chromatography. Mr. Houck has more than twenty issued and pending US and worldwide patents.
Food-Based Strategy for Nrf2-Active Plant Compounds.
Jed W. Fahey
His research addresses the induction by phytochemicals, of cytoprotective, anti-inflammatory, and antioxidant responses in mammalian systems. This work draws on elements of natural product chemistry, enzymology, nutritional epidemiology and clinical research to develop nutritional strategies for chronic disease prevention in humans. Many of these studies deal with the glucosinolates and isothiocyanates that are found primarily in cruciferous vegetables and in a nutritious tropical tree called the drumstick tree or Moringa oleifera.
Dr. Fahey’s group has developed, characterized, and supplied preparations rich in specific phytochemicals for a large number of animal and clinical studies in which they have played an integral collaborative role. Dr. Fahey also taught graduate courses in both the School of Public Health and School of Medicine. Before joining the JHU faculty in 1993, he spent 15 years in the biotechnology industry and held senior management positions in research and process development.
Dr. Fahey maintains adjunct faculty appointments at Johns Hopkins University School of Medicine, and the University of Maine Institute of Medicine. He now spends most of his time working to promote behaviors that enhance healthspan. His ongoing outreach work seeks to translate the science that his group and others have done to prevent chronic diseases, to a lay public audience. He may be available to consult for socially responsible food and supplement companies and foundations.

Jed W. Fahey. Dr. Fahey is a nutritional biochemist with broad training and extensive background in plant physiology, human nutrition, phytochemistry and nutritional biochemistry. He spent 27 years as a faculty member at the Johns Hopkins School of Medicine. Until retiring in mid-2020, he ran the Cullman Chemoprotection Center, which he helped to create, and which has for many years developed plant-based agents for the purpose of enhancing healthspan.
Skyclarys: Overview of its Discovery and Clinical Development in Friedreich Ataxia
Deborah Ferguson
Friedreich ataxia is a rare genetic neurodegenerative disease caused by a mutation in the frataxin (FXN) gene, which results in insufficient amounts of frataxin protein. Frataxin is involved in the assembly of iron-sulfur clusters, which are essential components of many enzymes involved in electron transfer and redox reactions. Frataxin deficiency is associated with dysregulated Nrf2 activity, mitochondrial dysfunction, oxidative stress, inflammation, and dysregulated iron metabolism. Skyclarys (omaveloxolone) is a semi-synthetic triterpenoid and a potent activator of Nrf2, a transcription factor that normalizes mitochondrial metabolism, restores redox balance, and promotes the resolution of inflammation. Skyclarys has been approved in the US for the treatment of patients with Friedreich ataxia age 16 years and older. In cells from patients with Friedreich ataxia, omaveloxolone has been shown to increase Nrf2 activity, reduce oxidative stress, and rescue mitochondrial dysfunction. In clinical trials, omaveloxolone significantly improved neurological function as assessed by modified Friedreich ataxia rating scale (mFARS) scores. In this talk, an overview of the discovery and development of omaveloxolone will be presented.

Deborah Ferguson is the Head of Scientific Communications for Nrf2 & Hsp70 Biology, having recently joined Biogen after twenty years with Reata Pharmaceuticals. During her time at Reata, she served in several roles with increasing responsibility and provided strategic scientific direction for the evaluation and development of investigational drugs, including Skyclarys and other Nrf2 activators. Prior to joining Reata, Deborah completed her PhD in Experimental Medicine at McGill University and a postdoctoral fellowship at UT Southwestern Medical Center.