April 20th
- 9:30 - 10:00 Registration.
- 10:00- 13:00. Management Meeting Only for MC members
- 13:00 - 14:00 Lunch / Registration
- 14.00-16:00. Session I. Chair: Ana Čipak Gašparović
NRF2 and the cancer cell: the cross-road of epigenetic networks and metabolic remodeling
Koraljka Gall Trošelj
Laboratory for Epigenomics, Division of Molecular Medicine, Rudjer Boskovic Institute,
Zagreb, CROATIA
Email: troselj@irb.hr
There are many published data related to NRF2 function associated with oxidative stress. However, its strong potential for regulating metabolic cellular processes, directly and indirectly, does not receive a well-deserved attention. Similarly so, epigenetic mechanisms involved in regulation of activity of the NRF2 coding gene (NFE2L2) are not common topic in the scientific community. However, both biological phenomena have important roles which impact NFE2L2 expression activity, which is considered to be universally beneficial for the cell. However, cellular protective mechanisms orchestrated through NRF2 activity, which are beneficial for the cell and the host in a setting of chemoprevention, remain beneficial only for the cancer cell but not for the host, once the malignant disease is developed. These facts deserve to be discussed in a setting of nutrient availability, keeping in mind that: a) cancer cell highly relies on glucose and glutamine; b) cancer cells supplied with sufficient amount of glucose tend to be chemoresistant. The potential role of NRF2 in these processes will be thoroughly discussed.

Prof. Koraljka Gall Trošelj, M.D.; Ph.D. is a senior research scientist at the Rudjer Bošković Institute where she established and has been heading Laboratory for Epigenomics. Dr. Gall Trošelj is a Fulbright Scholar who spent two years at the Weill College of Medicine at the Cornell University in New York City. She is the author and co-author of numerous articles, co-Editor of Frontiers of Oncology, Frontiers of Pharmacology and Cancer Cell International. She has been reviewer for many journals in the field of molecular and translational oncology. Her primary research interest is focused on basic research in oncology and functional connections between epigenomic networks and activity of cancer metabolism-related genes. She was also an international adviser and permanent secretary of ICMAN conferences (International Conferences on Mechanisms of Action of Nutraceuticals), which she organized in North Carolina, Israel, Australia and Scotland. She also gave many invitation lectures, in Croatia and abroad.
Nrf2 depletion leads to impaired mitochondrial respiration and mitolysosome accumulation:
Sharadha Dayalan Naidu1, Plamena R. Angelova2, Andrey Y. Abramov2, and Albena T. Dinkova-Kostova1
1 Jacqui Wood Cancer Centre, Division of Cellular and Systems Medicine, School of Medicine, University of Dundee, Dundee, UK
2 UCL Queen Square Institute of Neurology, Queen Square, London, UK
Email: a.dinkovakostova@dundee.ac.uk
Transcription factor nuclear factor erythroid 2 p45-related factor 2 (Nrf2, gene name NFE2L2) is frequently downregulated in ageing and disease, rendering cells and organisms more susceptible to the negative consequences of environmental challenges, and this downregulation is an important contributor to the pathogenesis of a wide range of chronic diseases. Using cells with a loss-of-function mutation in the main negative regulator of Nrf2, Kelch-like ECH associated protein 1 (Keap1), and their Nrf2-knockout or Nrf2-knockdown counterparts, we found that Nrf2 deficiency decreased mitochondrial respiration, but unexpectedly, increased the mitochondrial membrane potential, mass, DNA content, and mitolysosomes. The increase in mitolysosomes was particularly surprising, initially suggesting that mitophagy was enhanced in the absence of Nrf2, and in apparent conflict with the previously described decrease in LC3B lipidation and autophagic flux in Nrf2-deficient cells. Indeed, Nrf2 deficiency decreased the lipidation of LC3B. Moreover, Nrf2 deficiency promoted cysteine oxidation in ATG7 and ATG3, consistent with the critical role of Nrf2 in maintenance of the cellular redox homeostasis. However, the proportion of ATG7/ATG3 within their respective LC3B conjugates, was not decreased, but increased. Together with the increased number of mitolysosomes, mitochondrial mass and DNA, and the decreased LC3B lipidation, these findings imply that mitophagy is impaired at its late stages, leading to accumulation of damaged mitochondria within mitolysosomes that cannot be efficiently degraded.

ALBENA DINKOVA-KOSTOVA is a Professor of Chemical Biology at the University of Dundee School of Medicine (UK). She graduated in Biochemistry and Microbiology from Sofia University (Bulgaria) and obtained her PhD degree in Biochemistry and Biophysics from Washington State University (USA). She subsequently trained in Pharmacology at Johns Hopkins University School of Medicine (USA), where she continues to hold an Adjunct Professor position. She joined the University of Dundee in 2007 as a Research Councils UK Academic Fellow and a research group leader. Her group collaborates with basic scientists and clinicians,and with the pharmaceutical industry. In her research, at the interface of Chemical Biology and Medicine, she is committed to understanding how cells and organisms respond to oxidative, inflammatory, and metabolic stress, and is working towards development of strategies for protection against chronic disease. She was named among the top influential academics in Clarivate’s Highly Cited Researchers 2019, 2020, 2021 and 2022 lists.
Autophagy activation can partially rescue proteasome dysfunction-mediated cardiac toxicity
Eleni-Dimitra Papanagnou, Sentiljana Gumeni, Alexandra Rafeletou, Ioannis P. Trougakos*
1 Department of Cell Biology and Biophysics, Faculty of Biology, National & Kapodistrian University of Athens, Greece
Email: itrougakos@biol.uoa.gr
Protein quality control maintains proteome homeodynamics (proteostasis) and is critical for cellular functionality and viability especially for post-mitotic cells like cardiomyocytes, which are constantly exposed to proteotoxic, metabolic and mechanical stress. Proteostasis is assured by an integrate system called proteostasis network (PN), major components of which are the main degradation pathways i.e., the autophagy-lysosome and the ubiquitin proteasome pathways. Aberrant activation of the proteostasis mechanisms is found in advanced tumours and thus their inhibition provides promising anti-cancer therapies. We report that pharmacologically- or genetically- induced proteasome dysfunction in flies’ (Drosophila melanogaster) heart, leads to increased proteome instability and disrupted mitostasis, resulting in perturbation in cardiac functionality, systemic toxicity, and reduced longevity. These phenotypes were partially rescued by either heart targeted- or by dietary restriction-mediated autophagy enhancement. Supportively, Metformin or Rapamycin (autophagy activators) administration mitigated the toxic effects caused by targeted proteasome malfunction in heart by restoring proteome instability and mitochondrial functionality. These data represent a relevant preclinical insight for alleviating cardiovascular complications due to proteasome impairment.

Ioannis Trougakos obtained his Ph.D. in Cellular-Developmental Biology from the National and Kapodistrian University of Athens (NKUA), Greece. He has worked as Research Scientist at EMBL, Germany, CBM “Severo Ochoa”, Spain and at NHRF, Athens, Greece; he was also research visitor at EMBL and at the Netherlands Cancer Institute. Dr. Trougakos was elected Research Lecturer at NHRF and currently he serves as Professor and Director of the “Cell Biology” lab at the Faculty of Biology, NKUA. Dr. Trougakos has published articles (>190) in high-ranking journals, chapters in international books and he co-authored an academic book (citations ~19500; h-Index = 45 / i10-index = 129); he is also co-inventor in several patents. His group is funded by private (GR, EU, USA) and public (GR, EU) entities; also, the group participates in contractual activities with the Industry.
Nrf2 on the balance between redox biology and energy production
Andrey Y. Abramov
Department of Clinical and Movement Neurosciences, UCL Queens Square Institute of Neurology, Queen Square, London, UK
Email: a.abramov@ucl.ac.uk
Nrf2 is plays the role of the major controler of endogenous antioxidant pathways, in detoxification and also supports mitochondrial metabolism by substrates. The major cell protective strategy in various diseases by Nrf2 activation is based solely on the antioxidant effects. However, activation of the Nrf2 leads to upregulation of NADPH oxidases and increases ROS production in mitochondria. Energy metabolism and GSH level in brain cells are supported by Nrf2 through activation of of glucose uptake followed by higher production of NADPH and NADH. We have found out that inhibition of the glucose consumption in penthose phosphate pathway can lead to increased activity in energy metabolism. However, inhibition of energy metabolism does not lead to activation of PPP. Most of neurodegenerative disorders are associated with changes in mitochondrial metabolism and oxidative stress. Using Nrf2 activators in the cellular models of familial forms of Parkinson’s disease and ALS we found that restoration of the energy metabolism in these cells can be a major mechanism of neuroprotection. However, the combinations of the Nrf2 activation with inhibition of the ROS overproduction (NOX inhibitors) have sinergic effect on neuroprotection in Parkinson’s disease and ALS. Thus, Nrf2 is one of the most promising target for treatment of the neurodegenerative disorders.

Andrey Y. Abramov is a Professor at the Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology. He studies the role of mitochondria, calcium signalling and redox biology in physiology of the Central nervous system and in the mechanism of the pathology of neurodegenerative disorders.
Hypothermia Alleviates Reductive Stress, a New Concept in Ischemia Reperfusion Injury
Kattri-Liis Eskla1,2,a,*, Hans Vellama1,2,a, Liisi Tarve 1,2, Hillar Eichelmann3,4, Toomas Jagomäe1,2, Rando Porosk5, Vello Oja4,b, Heikko Rämma4, Nadežda Peet3, Agu Laisk4, Vallo Volke3, Eero Vasar1,2, Hendrik Luuk1,2
1 Institute of Biomedicine and Translational Medicine, Department of Physiology, University of Tartu, Tartu, Estonia
2 Center of Excellence for Genomics and Translational Medicine, University of Tartu, Tartu, Estonia
3 Institute of Biomedicine and Translational Medicine, Department of Pathophysiology, University of Tartu, Tartu, Estonia
4 Institute of Technology, University of Tartu, Tartu, Estonia
5 Institute of Biomedicine and Translational Medicine, Department of Biochemistry, University of Tartu, Tartu, Estonia
a These authors contributed equally
b Deceased
* Corresponding author
Email: kattri-liis.eskla@ut.ee
Aims. Ischemia reperfusion injury is a pathological process that is the most common cause of organ transplant rejection/failure. Cooling has proven to be the first line of defense against hypoxic injury. We propose here that the widely accepted view that hypothermia generates a state of metabolic depression is most likely incomplete. To the best of our knowledge, no attempts have been made to uncover a mechanistic explanation as to why mild hypothermia (32 °C) is therapeutically more efficient than moderate (28 °C) and deep (22 °C) hypothermia. The main idea of this study was to assess the effect of hypothermia on the activation of cellular stress pathways (the activating component) and the induction of metabolic suppression (the inhibitory component) at various temperatures.
Methods. Since kidney transplantation is one of the most common organ transplant surgeries, we used human-derived renal proximal tubular cells (HKC-8 cell line) as a model of normal renal cells. We performed temperature titration curve from 37°C to 22°C under normoxia or hypoxia (1% O2) followed by different downstream applications like biochemistry, molecular biology, and novel cellular gas flux system for measuring CO2 and O2 flux. It allows us to measure the production (by CAC) and utilization (by ETC) of reducing power.
Results. We show that the effects of hypothermia are likely to stem both from metabolic suppression (inhibitory component) and augmentation of stress tolerance (activating component) with the highest optimal activation of both components emerging in the window of mild hypothermia (32°C). Hypothermia decreased hypoxia-induced rise in the extracellular lactate:pyruvate ratio, increased ATP/ADP ratio and mitochondrial content, normalized lipid content and improved the recovery of respiration after anoxia. Importantly, it was observed that in contrast to mild hypothermia, moderate (28°C) and deep hypothermia (22°C) interfere with HIF1-dependent HRE induction in hypoxia. Our work also demonstrates that hypothermia alleviates reductive stress, a conceptually novel and largely overlooked phenomenon at the root of ischemia reperfusion injury.
Conclusion. This study addresses an important question on why hypothermia is effective in reducing hypoxic tissue damage. Current evidence highlighting potentially therapeutic molecular mechanisms can be translated to in vivo models, for further evaluation of therapeutic efficacy. More importantly, the reductive stress hypothesis bears the promise of opening up a new view on potential treatment strategies.

Kattri-Liis Eskla. In 2019, Kattri-Liis graduated her PhD in neuroscience at University of Tartu (Estonia) and discovered the profound influence that hypothermia has on the antioxidant system. During her internship in Emory University School of Medicine (USA), she studied hydrogen sulfide and therapeutic lymphangiogenesis as promising new approaches for the treatment of cardiovascular diseases. In 2021, she began as a visiting researcher at the University of Birmingham (UK) to undertake a collaborative research project on the hypoxic and pseudohypoxic biology and cellular metabolism. In 2020, she became a research fellow in human physiology at the Department of Physiology, University of Tartu, Estonia. Her work has broader implications challenging the status quo in the field of hypothermia. She proposes that the widely accepted view that metabolic depression is mostly accountable for the therapeutic effects of hypothermia is most likely incomplete. Her main focus is understanding mechanisms of hypothermia and reductive stress which will lead to enhanced treatment of hypoxic/ischemic conditions as well as other disorders associated with electron transport chain dysfunction and reactive oxygen species formation.
- 16:00 - 16:30 Coffee break.
- 16.30-18:30. Session II. Chair: Antonio Cuadrado
Is there a Link Between NRF2 and Depression?
Marlene Santos 1,3* Debora Fonseca1, Renato Caldevilla 1,2, M. Fátima Barroso 2, and Agostinho Cruz
1 CISA|ESS, Centro de Investigação em Saúde e Ambiente, Escola Superior de Saúde, Polytechnic Institute of Porto, Rua Dr. António Bernardino de Almeida, 400, 4200-072, Porto, Portugal;
2 REQUIMTE–LAQV, School of Engineering, Polytechnic Institute of Porto, R. Dr. António Bernardino de Almeida 431, 4200-072, Porto, Portugal;
3 Molecular Oncology and Viral Pathology Group, Research Center, Portuguese Oncology Institute of Porto – Francisco Gentil, R. Dr. António Bernardino de Almeida 865, 4200-072 Porto, Portugal;
Email: mes@ess.ipp.pt;
Introduction: Depression is a common mental health disorder that affects millions of people worldwide. Recent studies have highlighted the role of oxidative stress and inflammation in the pathogenesis of depression. NRF2 is a transcription factor that plays a crucial role in cellular defense against oxidative stress by binding to antioxidant response elements (AREs) located in the promoter region of various phase II antioxidant enzymes and stress-responsive enzymes. Decreased Keap1-Nrf2 signaling has been implicated in the development of mood disorders, such as Major Depressive Disorder. Therefore, this review aims to evaluate the in vitro and in vivo evidence of the involvement of Nrf2 in depression.
Methods: A review was conducted on the PubMed database for articles published until March 8, 2022 Papers that evaluated NRF2 in animals and/or cell lines with depression and were published in English were included in the review. Studies that addressed other diseases/topics, systematic reviews, and those that did not address NRF2 were excluded. Quality assessment was performed according to Koch et al., 2022.
Results: Out of the 203 possibly relevant abstracts found through the PubMed search, 45 papers were included in the review. The results suggest that Nrf2 levels tend to decrease in animals exposed to oxidative stress or depressive behavior. When animals were treated with antidepressants or anti-inflammatory drugs, Nrf2 levels increased. Additionally, the study found that IL-10 and BDNF were key elements that were positively influenced by Nrf2 levels, protecting against oxidative stress through Keap1/Nrf2.
Discussion and conclusions: The findings suggest that Nrf2 activation may play a crucial role in controlling oxidative stress and inflammation during depression. Furthermore, it provides evidence of the involvement of Nrf2 in depression and highlights its potential as a therapeutic target. However, further studies on clinical samples are necessary to evaluate NRF2’s putative effect in depression and antidepressant response.
Marlene Santos (Orcid: https://orcid.org/0000-0001-5020-5942) holds a PhD in Biomedicine, MSc in Molecular Genetics, and a Degree in Pharmacy. She is Adjunct Professor at the School of Health from Polytechnic Institute of Porto, Portugal, where she is the Director of the master’s degree in pharmacy. She is also Invited Investigator at Molecular Oncology and Viral Pathology Group of the Research Centre of the Portuguese Institute of Oncology of Porto, and investigator at Health and Environment Research Center (CISA) from Polytechnic Institute of Porto. Additionally, she has been involved in several national and international projects, as well as COST actions. Her research interests focus on the study of biomarkers of psychiatric and neurological diseases and treatment response outcomes, and her main publications are in the field of Pharmacogenomics and Neuropsychopharmacology.
Susceptibility of hepatic progenitor cells lo lipotoxicity and hypoxia: potential role of NRF2 in preserving cell fate
Laura Villamayor1,2, Inés Barahona1,2, Noelia Arroyo3, Silvia Calero1,2, Elena del Fresno1, Pedro M. Rodrigues4,5,6, Elena Carceller-López1, Malgorzata Milkiewicz7, Piotr Milkiewicz8,9, Jesús M. Banales4,5,6, Anabel Rojas2,3, Águeda González-Rodríguez1,2 and Ángela M. Valverde1,2.
1. Institute of Biomedical Research “Alberto Sols” (CSIC-UAM), 28029, Madrid, Spain.
2. Network Biomedical Research Center for Diabetes and Associated Metabolic Diseases (CIBERdem), 28029, Madrid, Spain.
3. Andalusian Molecular Biology and Regenerative Medicine Centre (CABIMER), University Pablo de Olavide, Universidad de Sevilla, Consejo Superior de Investigaciones Científicas (CSIC), Seville, Spain.
4. Department of Liver and Gastrointestinal Diseases, Biodonostia Health Research Institute, Donostia University Hospital, University of the Basque Country (UPV/EHU), San Sebastian, Spain.
5. National Institute for the Study of Liver and Gastrointestinal Diseases (CIBERehd, Carlos III Health Institute), Madrid, Spain.
6. IKERBASQUE, Basque Foundation for Science, Bilbao, Spain.
7. Department of Medical Biology, Pomeranian Medical University in Szczecin, 70-111 Szczecin, Poland.
8. Liver and Internal Medicine Unit, Department of General, Transplant and Liver Surgery, Medical University of Warsaw, 02-097, Warsaw, Poland.
9. Translational Medicine Group, Pomeranian Medical University, 70-204, Szczecin, Poland.
10. Department of Biochemistry and Genetics, School of Sciences, University of Navarra, Pamplona, Spain.
Activation of hepatic progenitor cells (oval cells in mice) has been related to hepatocyte injury during chronic liver diseases as a regenerative response to repair liver damage. Therefore, the plasticity of oval cells can be affected under pathological environments including lipotoxicity and hypoxia. In this study we have investigated, on the one hand, the impact of the treatment with palmitic acid (PA), a saturated FA highly lipotoxic and, on the other, the effects of low oxygen saturation, in oval cell fate. PA induced apoptotic cell death in oval cells in parallel to oxidative stress, mitochondrial damage and impaired autophagy. Likewise, nuclear and cytosolic NRF2 levels, expression of NRF2-target genes (Nqo1, Hmox1) and Heme oxygenase (HO-1) and Catalase protein levels, showed that deficiency in Ptpn1, encoding protein tyrosine phosphatase 1 (PTP1B) upregulated NRF2-mediated antioxidant defences and triggered a rapid antioxidant response against lipotoxic stress. Treatment of oval cells with PA plus sulforaphane increased nuclear NRF2, enhanced antioxidant gene expression and reduced DHE staining and lipid peroxidation. As a result, apoptosis was ameliorated. On the other hand, oval cells exposed to 1% O2 showed features of epithelial-to-mesenchymal transition (EMT). At the molecular level, the up-regulation of hypoxia-inducible transcription factor HIF2α concurred with an elevation of nuclear NRF2, Hmox1 mRNA levels and NQO1 protein levels. In addition, we identified for the first time the zinc-finger transcription factor GATA4, a suppressor of hepatic stellate cells activation, in oval cells and, importantly, its expression decreased under hypoxic conditions. In vivo experiments showed oval cell expansion in mice with non-alcoholic fatty liver disease (NAFLD) and mice exposed to intermittent hypoxia. Finally, gene expression levels related to hepatic progenitor cells, obtained from the NCBI Gene Expression Omnibus database, were significantly increased in NAFLD patients with advanced fibrosis compared to those with mild fibrosis. Moreover, the expression levels of GATA4 in liver samples from patients with biliary diseases (primary sclerosing and primary biliary cholangitis) revealed a significant decrease in PSC patients and a decrease trend in PBC patients. In conclusion our results suggest that targeting NRF2 might be a therapeutic strategy to prevent the loss of oval cell fate during chronic liver diseases.

Ángela Martínez Valverde Instituto de Investigaciones Biomédicas Alberto Sols (CSIC-UAM) Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERdem). Instituto de Investigación Hospital Universitario La Paz (IdiPAZ).
My research career has been focused in molecular metabolism. As PhD fellow (1988-91), I studied insulin/IGF1 actions in brown adipocytes. As postdoc at Cancer Research UK (London, 1991-93), I cloned protein kinase D1, a Ser/Thr kinase that later studies revealed its fundamental role in cancer and recently, in glucose homeostasis. Back to Spain as Assistant Professor at Complutense University of Madrid, I implemented my expertise in signal transduction in unraveling insulin/IGF1 cascades related to proliferation/differentiation of brown adipocytes. From 2002 and as a Senior Scientist at Spanish National Research Council (CSIC), I explored the molecular mechanisms of insulin action in the liver. In 2006, I moved to the Institute of Biomedicine Alberto Sols (CSIC/UAM) where I consolidated my leadership as PI and extended my research to the molecular basis of obesity and non-alcoholic fatty liver disease (NAFLD).
From 2008, I am Principal Investigator at CIBERdem (Spanish network in research on diabetes and related metabolic diseases). In this topic, we studied the impact of insulin resistance, ER stress, autophagy, oxidative stress and inflammation in NAFLD and more recently in hepatic biliary diseases. A step further, we investigate the interactome between liver cells in the NAFLD and biliary disease context. We are currently extending this interactome to the extracellular vesicles field and hepatic progenitor cells. Regarding NAFLD therapeutics, we unravelled the efficacy of a GLP1/Glucagon receptor co-agonist in improving liver regeneration during NASH. In the last 10 years, our lab has also studied the molecular basis of the diabetic complications retinopathy and nephropathy, as well as drug-induced hepatotoxicity. In the context of metabolic alterations induced by chronic drug treatments, we evaluate how antipsychotic medication impacts on whole body metabolism and energy balance.
Genetic variability of NRF2 pathway and neurodegeneration
David Vogrinc*1, Sara Redenšek Trampuž*1, Katja Goričar1, Vita Dolžan1
1 Pharmacogenetics Laboratory, Institute of Biochemistry and Molecular Genetics, Faculty of Medicine, University of Ljubljana, Vrazov trg 2, SI-1000 Ljubljana, Slovenia
* Both authors contributed equally to the work
e-mail: vita.dolzan@mf.uni-lj.si
As oxidative stress contributes to neurodegeneration, transcription factor NRF2, its repressor KEAP1, and NRF2 target genes present an important signaling pathway in neurodegenerative diseases, especially Alzheimer’s disease (AD) and Parkinson’s disease (PD). NRF2 activity was shown to be reduced in AD patients. Additionally, it has a neuroprotective role in PD affecting microglial dynamics and astrocyte activation (1). Genetic variability of NFE2L2 and other genes from the signaling pathway has been associated with PD risk and its symptomatology (2,3). Preliminary results from our group suggest both NFE2L2 and KEAP1 genetic variability might be associated with cerebrospinal fluid biomarkers and symptomatology of AD. miRNA landscapes of the NRF2 pathway have also been associated with both AD and PD (4,5). Our pathway enrichment analysis suggested that miRNAs associated with neurodegeneration as a COVID-19 sequela might also regulate NRF2 signaling pathway and may have a valuable potential for neurodegeneration prediction and therapy in COVID-19 patients (6).

Vita Dolžan is a Full Professor of Biochemistry and Molecular Biology at the Faculty of Medicine, UL. She investigates the influence of genetic variability in drug metabolizing, antioxidative and inflammatory pathways on disease pathogenesis, disease progression and/or treatment response in several diseases, with particular focus on neurodegenerative and inflammatory diseases as well as cancer. She is particularly interested in development of clinical-pharmacogenetic models that would facilitate the translation of genetic, epigenetic and other molecular biomarkers into clinical practice.
References
1. Saha S, Buttari B, Profumo E, Tucci P, Saso L. A Perspective on Nrf2 Signaling Pathway for Neuroinflammation: A Potential Therapeutic Target in Alzheimer’s and Parkinson’s Diseases. Frontiers in Cellular Neuroscience. 2022;15.
2. von Otter M, Landgren S, Nilsson S, Celojevic D, Bergström P, Håkansson A, et al. Association of Nrf2-encoding NFE2L2 haplotypes with Parkinson’s disease. BMC Med Genet. 2010;11:36.
3. von Otter M, Bergström P, Quattrone A, De Marco EV, Annesi G, Söderkvist P, et al. Genetic associations of Nrf2-encoding NFE2L2 variants with Parkinson’s disease – a multicenter study. BMC Med Genet. 2014;15:131.
4. Paladino S, Conte A, Caggiano R, Pierantoni GM, Faraonio R. Nrf2 Pathway in Age-Related Neurological Disorders: Insights into MicroRNAs. Cell Physiol Biochem. 2018;47(5):1951-76.
5. Xie Y, Chen Y. microRNAs: Emerging Targets Regulating Oxidative Stress in the Models of Parkinson’s Disease. Front Neurosci. 2016;10:298.
6. Redenšek Trampuž S, Vogrinc D, Goričar K, Dolžan V. Shared miRNA landscapes of COVID-19 and neurodegeneration confirm neuroinflammation as an important overlapping feature. Front. Mol. Neurosci. 2023: 16:1123955.
Air Pollution Exposure induces NRF2 in Astrocytes
Saveleva L1, Ivanova M1, Afonin A1, Jalava P2, Kanninen KM 1
1 A.I.Virtanen institute for Molecular Sciences, University of Eastern Finland
2 Dept of Environmental and Biological Sciences, University of Eastern Finland
Email: Katja.Kanninen@uef.fi
Outdoor air pollution is the largest environmental risk factor that has been associated with cardiovascular, lung, and lately also neurodegenerative diseases. Prior work has demonstrated that air pollutant exposure can lead to neuroinflammation, oxidative stress and the appearance of protein aggregates in the brain. Evidence suggests that airborne particles can enter the brain directly through olfactory nerve or enter the blood circulation. However, there is an unmet need for understanding how different brain cell types are involved in the adverse effects of air pollutant exposure. In this study, we aimed to decipher how size-segregated particulate matter (PM) that was collected from urban air in Nanjing, China affects the viability and transcriptome of adult astrocytes. We used mouse primary astrocytes harvested from the adult mouse brain of C57BL/6J mice, which at 21 days in culture were exposed for 24 hours to nanosized (less than 0.2 µm) particulate matter (nPM) collected from urban air. Cell viability and mRNAsequencing data obtained demonstrate that nPM trigger activation of antioxidative stress signalling and detoxification systems in adult astrocytes without affecting cell viability, indicating activation of the cellular protection system in response to nPM. Overall, we found a total number of 2217 genes to be differently expressed when nPM-exposed astrocytes were compared to vehicle control. The most activated canonical pathways after exposure were xenobiotic metabolism and the NRF2 oxidative stress response pathways. We suggest that polycyclic aromatic hydrocarbons that are present in airborne particles trigger activation of xenobiotic metabolism and NRF2 signaling, leading to protective activation of antioxidant genes in astrocytes. These results provide new insight into astrocyte responses to air pollutant exposure.

Katja Kanninen is a professor of Cellular Neurobiology at the A.I.Virtanen Institute for Molecular Sciences, University of Eastern Finland. She defended her thesis on NRF2 in Alzheimer’s disease and is now interested in understanding the interplay between environmental exposures such as air pollutants and NRF2. Her group works with in vivo models of neurodegeneration, and with both human and murine brain cells (primarily astrocytes and neurons), and human olfactory cells. She has published several original and review papers on NRF2, primarily in the context of Alzheimer’s disease.
Repositioning dimethyl fumarate for the treatment of TDP-43-dependent frontotemporal dementia
Lastres-Becker, I.1,3, Berrojo-Armisen, A.1*, Martín -Baquero, R.2,3*, Fernández-Ruiz, J. 2,3, De Lago, E. 2,3
1 Instituto de Investigaciones Biomédicas “Alberto Sols” UAM-CSIC, Arturo Duperier, 4, Department of Biochemistry, School of Medicine, Institute Teófilo Hernando for Drug Discovery, Universidad Autónoma de Madrid , 28029 Madrid, Spain.
2 Instituto Universitario de Investigación en Neuroquímica, Departamento de Bioquímica y Biología Molecular, Facultad de Medicina, UCM, Madrid, Spain, Instituto de Salud Carlos III, Spain, Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Madrid, Spain.
3Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED)
* have participated equally in the work
Email: ilbecker@iib.uam.es
Frontotemporal dementia (FTD) is an early-onset, progressive neurodegenerative disease characterized by primarily neuronal degeneration in the frontal and temporal lobes followed by hippocampal atrophy. After Alzheimer’s disease (AD), FTD is the second cause of dementia in adult patients and the most frequent in patients under 65 years of age. From a histopathological and molecular point of view, FTD is a proteinopathy involving the dysregulation (e.g. loss of function, aggregation) of two main proteins: i) the cytoskeleton-related protein TAU or (ii) the ribonucleoprotein TDP-43. There is also dysregulation of redox homeostasis and low-grade chronic neuroinflammation. Currently, there is no approved effective treatment for FTD that could slowdown the course of the disease. Recently, we identified the transcription factor NRF2 as a key factor to limit neurodegenerative process in other diseases. NRF2 has pleiotropic effects by acting on various molecular mechanisms involved in the neurodegenerative process. Within the brain, it has been described that pharmacological activation of NRF2 has beneficial effects against protein aggregates, neuroinflammation, and oxidative stress. Studies carried out by our group demonstrated neuroprotective effects of the NRF2 activator dimethyl fumarate (DMF) in a TAU-dependent FTD model, reducing TAU hyperphosphorylation, neurodegeneration, neuroinflammation and oxidative stress, supporting the reposition of this drug for the treatment of FTD. Furthermore, we observed that TAU overexpression delocalizes TDP-43 from the nucleus to the cytoplasm, and DMF treatment is able to relocate it to the nucleus, indicating an interconnection between TAU and TDP-43. However, the possible efficacy of this treatment in TDP-43-FTD is not known yet. Therefore, in this work we have evaluated the possible neuroprotective effects of DMF treatment in a TDP-43-FTD mouse model as CaMKII-TDP-43-transgenic mice. These mice overexpressed TDP-43 only in the forebrain, mimicking the characteristic phenotype of FTD with ubiquitin-positive inclusions (FTD-U).

Isabel Lastres-Becker has more than 20 years of experience in the field of neurodegenerative diseases. Her work is focused on the molecular bases of neurodegeneration related to proteinopathy, neuroinflammation, and oxidative stress. She is the head of the laboratory of “New therapeutic strategies in neurodegenerative diseases: Parkinson’s disease (PD), tauopathies and amyotrophic lateral sclerosis (ALS)”. Since 2008 she has been interested in the implication of the transcription factor NRF2 in neurodegeneration, endorsed by 20 publications in relevant international journals. Her wide research background covers the most prevalent neurodegenerative diseases, looking for reliable markers of progression that can also serve as drug targets for modulating neurodegeneration such as NRF2. She is engaged in developing advanced new drugs and appropriate technology to establish treatments for those diseases, based on NRF2.
Repurposing TXA302 as a novel senotherapeutic
Ognian Neytchev1, Colin Selman2 and Paul G. Shiels1
1 School of Molecular Biosciences, MVLS, University of Glasgow
2 School of Biodiversity, One Health and Veterinary Medicine MVLS, University of Glasgow
email: paul.shiels@glasgow.ac.uk
Ageing is a risk factor for multiple chronic diseases, which is both malleable and druggable, using established pharmaceuticals or known naturally occurring bioactive substances, that can mitigate the common effects of the dysregulation of the ageing process. In particular, many of these agents exert their health beneficial effects via Nrf2 agonism. We have therefore investigated repurposing the capacity of the Angiotensin 1-7 analogue TXA302, a clinical anti-stroke agent, which has been shown to provide a senotherapeutic effect, the mechanistic basis of which remains undetermined.
We now report on the potential of TXA302 to act as a senotherapeutic agent capable of slowing the rate of organismal ageing and improving health span in a murine model of normative ageing. To assess health span, a range of physiological and molecular markers were measured: grip strength, glucose tolerance, inflammation (IL-6), Nrf2 expression, gut microbiome diversity, telomere length, DNA methylation age, and expression of the CDK inhibitors CDKN1A, CDKN2A, and CDKN2B in a cohort of treated mice (n=102) and untreated controls (n=102). TXA302 was well tolerated and improved several parameters subject to age-associated decline, enhancing grip strength and glucose tolerance, while reducing inflammation, cellular senescence, and increasing gut microbial diversity over the life course of the mice, with no adverse effects detected. Notably, we observed no changes in epigenetic age as measured by a DNAm clock, nor in Nrf2 activity. The implications of these findings will be discussed.

Paul Shiels. Paul is Professor of Geroscience at the University of Glasgow. He is a graduate of Trinity College Dublin and received his PhD from Glasgow University. He won an EMBO Long-term Fellowship to work at the Netherlands Cancer Institute. This was followed by a period at the Robertson Center for Biotechnology, before joining PPL Therapeutics, Roslin (1996) where he worked on senescence in cloned animals. He is a founder member of G3, the Glasgow Geroscience Group. Paul has established a reputation in the field, developing the kidney as a model of ageing, and is author of over 198 peer reviewed publications and a number of Patents in this sector. Paul has acted as an expert on the Biology of Ageing on national policy advising consortia including pSoBiD, for the Chief Medical Officer of Scotland, and provided evidence to the UK All Party Parliamentary Group on Longevity. He is currently Chair of the Scientific Advisory Board for the British Society for Research on Ageing. His research has involved determining exposome factors (socio-economic, psychological, lifestyle and nutrition) and (epi)genomic factors that are required for healthy ageing. He was the first to report on socioeconomic status and nutritional factors affecting epigenetic influences on health. His current research portfolio comprises investigation and application of novel senotherapies, biomimetics and how the microbiome impacts on age related health.
Paul has acted as CSO for Pathfinder Cell Therapy PLC and has been funded by, acted as a consultant for and sat on the Scientific Advisory Boards of a wide range of Pharma companies. He has a proven track record in public dissemination of his research including the provision of expert commentary for the BBC and ABC TV networks and as a Panelist at the Edinburgh International Science Festival and the Edinburgh International Book Festival.
- Evening, starting at 18:30. Join WG meetings Chair: WG leaders
April 21th
- 9:00 - 11:00. Session III. Chair: Dr Christina Morgenstern
Role of Nrf2 signaling in the crosstalk between Trx1 and GSNOR-redox regulators in liver cancer cells
Silvia Silva-Hucha1,2, Elena Navarro-Villarán1,2,3, Natalia García-Villasante1, Mª Belén Maqueda- Hernández1, Salvatore Rizza4, Giuseppe Filomeni4.5, Jordi Muntané1,2,3
1Institute of Biomedicine of Seville (IBIS), Hospital University Virgen del Rocío/CSIC/University of Seville, Spain.
2Department of Medical Physiology and Biophysics, University of Seville, Seville, Spain.
3Biomedical Research Center for Hepatic and Digestive Diseases (CIBERehd).
4Redox Biology Group, Danish Cancer Society Research Center, Copenhagen, Denmark. 5Department of Biology, University of Rome Tor Vergata, Rome, Italy.
5Center for Healthy Aging, University of Copenhagen, Denmark.
Email: ssilva@us.es
Introduction: Hepatocellular carcinoma (HCC) constitutes the sixth most common type of cancer and the second leading cause of death by cancer. Atezolizumab+Bevacizumab and Sorafenib are first-line treatments for patients in advanced HCC. The proapoptotic and antiproliferative properties of Sorafenib activities are associated with altered cellular redox status, and downregulation of Nrf2-related thioredoxin-1 (Trx1) expression and activity in liver cancer cells. S-nitrosoglutathione reductase (GSNOR) is involved in the regulation of cellular S-nitrosation process. Aim: Identification of the crosstalk among redox and S-nitrosation regulations in liver cancer cells during Sorafenib treatment in cancer cells. Material and Methods: We determined the impact of Nrf2 activator (sulforaphane) and/or regulation of GSNOR expression by siRNA in cell death and proliferation, cell migration and invasiveness by Sorafenib in 2D cultured liver cancer cells. Sorafenib (10 µM) was administrated to HepG2. shSCR- and shGSNOR-transfected HepG2 cells were used to elucidate the role of GSNOR, and Nrf2 was induced using Sulforaphane (10 µM) in HepG2 cells. We assessed GSNOR, Nrf2, Trx1, NQO1, vimentin, β-cadherin, N-cadherin and slug expressions by Western-blot analysis at different time points (0-24 hours). Apoptosis and cell proliferation were determined using caspase-3-related substrate and BrdU incorporation, respectively. We performed cell migration and invasiveness analysis at different time points (0-48 hours). Results: The reduction of cell proliferation, migration and invasiveness by Sorafenib was related to downregulation of Nrf2 and GSNOR protein expression. Sulforaphane increased Trx1 and NQO1 protein expression in HepG2. Interestingly, although the activation of Nrf2 by Sulforaphane transiently increased mRNA GSNOR expression, its protein content was reduced by Sulforaphane in control cells, Sulforaphane increased cell migration and invasiveness in control cells. Sorafenib was able to prevent the increase of cell migration and invasiveness until 36 h of treatment. Interestingly, the sustained Sulforaphane administration over 36 h overcame Sorafenib-antitumoral properties. Conclusions: Sorafenib reduced Nrf2-dependent signaling and GSNOR expression in liver cancer cells. The regulation of GSNOR expression by Sulforaphane suggests a relevant crosstalk between redox regulation and nitrosative stress. Sustained Sulforaphane treatment overcame antitumoral properties of Sorafenib.

Silvia Silva-Hucha: I obtained my PhD in neurosciences at the University of Seville (2020). Afterwards, I joined the Professor Steve Hunt’s group at the University College London with a project funded by the Medical Research Council. My pre and postdoctoral career has given me experience in the study and treatment of neurodegenerative diseases, and the important role of the VEGF trophic factor in establishing differential resistance to motor neurons. Last March 2023. I joined the Jordi Muntané’s lab in the Institute of Biomedicine of Seville to elucidate the molecular mechanisms of action of first line therapies for the management of patients with advanced HCC.
Screening natural compounds for effects on Nrf2 and the thyroid
Panos G. Ziros1, Georgios Psarias1, Dionysios V. Chartoumpekis1, Gerasimos P. Sykiotis1*
1 Service of Endocrinology, Diabetology and Metabolism; Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
Recent studies have identified pleiotropic roles for the Nrf2 antioxidant response in the physiology of the thyroid gland and in the pathophysiology of various thyroid diseases. Various natural compounds have antioxidant properties and/or thyroidal effects, but it is not well known whether and how the two are related. The aim of the present study was to characterize in a systematic manner the thyroidal and antioxidant effects of natural compounds. We performed a low-throughput manual chemical screen of >400 natural compounds in thyroid follicular cell lines stably transfected with reporter constructs. We used the rat PCCL3 cell line in its wild-type form as well as a PCCL3 Nrf2-knockout clone generated via CRISPR/Cas9 mutagenesis. The following read-outs were assessed: Nrf2 transcriptional activity (ARE-luciferase); Nrf1 protein stabilization (Nrf1-delta-luciferase); cell viability (CellTiter-Glo); reporter gene expression of the thyroid hormone precursor thyroglobulin (TG-luciferase); reporter gene expression of the sodium-iodide symporter (NIS-luciferase); and iodine uptake by the cells. For compounds showing activity in the respective assays, the mRNA and protein levels of NIS, TG and the antioxidant gene Nqo1 were assayed by qRT-PCR and Western blot, respectively. We identified several compounds showing activity in one or several assays. Among other findings, we observed that certain compounds paradoxically induced higher transcriptional activation of the ARE in Nrf2-knockout cells than in wild-type cells. Further studies showed that these compounds were potent activators of Nrf1, which is highly expressed in the thyroid in vivo. In a separate set of results, the flavonoid compound bavachin was found to increased iodine uptake by the cells in an Nrf2-independent manner. Importantly, bavachin was also able to reverse the decrease in iodine uptake that is induced by exposure to lithium, a drug used in the treatment of bipolar disorder. In conclusion, screening of natural compounds in thyroid cell lines can yield relevant hits with potential therapeutic relevance in thyroid diseases.

Gerasimos (Gerry) Sykiotis is Senior Staff Physician at Lausanne University Hospital and Associate Professor at the University of Lausanne. He specializes in clinical and basic endocrinology with a particular focus on thyroid physiology and thyroid diseases, including thyroid cancer. Since 2015, he is responsible for the thyroid clinic at the Endocrine Division of Lausanne University Hospital. His basic research, funded primarily by the Swiss National Science Foundation, focuses on the roles of cellular antioxidant response systems in thyroid physiology and pathophysiology. His clinical research focuses on the needs of patients with benign and malignant thyroid diseases.
Synthesis of new electrophilic NRF2 activators
Srđan Bjedov
Department of Chemistry, Biochemistry and Environmental Protection, Faculty of Sciences, University of Novi Sad, Serbia
e-mail: srdjan.bjedov@dh.uns.ac.rs
The NRF2 is a well-established pharmacological target for the treatment of a number of non-communicable diseases such as inflammatory, neurodegenerative, metabolic, and cancer diseases. The FDA approved a very potent, electrophilic NRF2 activator, Omaveloxolone, in February of 2023 for the treatment of Friedreich ataxia. The triterpenoid structure of Omaveloxolone is closely related to steroids, a class of molecules with a biologically privileged structure. Synthesis of new, potential NRF2 activators based on the steroidal skeleton and some biological properties of these compounds will be presented.
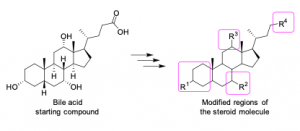

Srđan Bjedov is an assistant professor at the Department of Chemistry, Biochemistry and Environmental Protection, Faculty of Sciences, University of Novi Sad, Serbia. His research is focused on the synthetic and medicinal chemistry of mainly steroid compounds for the treatment of inflammatory, microbial, and cancer diseases.
Testing stimulatory NRF2 compounds that can reduce lipofuscin accumulation in RPE cell models
Ana S Falcão, Margarida Pedro, Vicente Miguel, Tomás Guerreiro, Rita Coelho, Sandra Tenreiro and Miguel Seabra
iNOVA4Health, NOVA Medical School|Faculdade de Ciências Médicas, NMS|FCM, Universidade Nova de Lisboa; 1169-056 Lisboa, Portugal.
Email: ana.falcao@nms.unl.pt
Age-related macular degeneration (AMD) is the most common blinding disease in the western world and is currently incurable. One of the main pathologic features of AMD is the retinal pigment epithelium (RPE) degeneration as well as the accumulation of autofluorescence (lipofuscin). We have developed a model system that recapitulates some AMD features in vitro where feeding human RPE monolayers in culture with porcine photoreceptor outer segments (POS) leads to accumulation of autofluorescent granules similar to lipofuscin in vivo, mimicking RPE cellular stress and disease. Interestingly, our preliminary results in RPE cells showed that overexpression of the transcriptional regulator NRF2, as well as treatment with dimethyl fumarate (DMF), a known NRF2 activator, can prevent the formation and/or resolve POS derived autofluorescent granules, suggesting that this pathway and the proteins it controls are important for autofluorescent granule formation. Thus, our hypothesis is that induction of an anti-oxidative stress response could represent a therapeutic strategy in early/intermediate AMD.
Focusing on repurposing clinically approved drugs that are described to be pharmacological modulators of NRF2, such as Curcumin, Bardoxolone methyl and Sulforaphane, we tested these compounds in our RPE AMD in vitro model, and evaluate their ability to decrease autofluorescent granules formation, as compared with DMF. Our first results point out Curcumin as an emerging pharmacological approach to decrease the accumulation of autofluorescence. Further studies will also include human phenolic metabolites library screening of NRF2 activators. We are also validating protocols for NRF2 activation in RPE cells by using western blot, immunofluorescence and gene expression assays.
Overall, this project could represent a major step forward, not only for AMD treatment but also for other age-related diseases, and consequently, will contribute to a healthier ageing.
Project funded by “La Caixa Foundation” (NASCENT HR22-00569)

Ana Sofia Falcão holds a PhD in Pharmacy since 2006 and she is currently working as a post doctoral researcher at the Molecular Mechanisms of Disease Lab, at Nova Medical School (NMS), from Universidade Nova de Lisboa. At NMS, she became interested in exploring innovative therapeutic approaches for neurodegenerative disorders or other age-related diseases, such as AMD. She is focused on in vitro systems to study retinal pigment epithelial cells that will be used for fundamental studies as well as for potential translational applications to treat retinal disorders.
Carbon nanoparticles for targeted drug delivery
Silvia Giordani
1School of Chemical Sciences, Dublin City University, Glasnevin, Dublin 9, Ireland
Email: silvia.giordani@dcu.ie
There are many issues associated with free drug delivery—the most prominent of which include: adverse side-effects, multi-drug resistance, premature drug degradation, lack of tissue penetration, and non-specific toxicity. Targeted delivery, which utilises nanocarriers as payload delivery vesicles, has the potential to address and alleviate these prominent issues. Specifically, it involves nanomaterials functionalised with targeting agents, allowing for the selective uptake of these nanocarriers by cells overexpressing specific receptors. This approach explicitly increases the drug concentration in the target cell of interest whilst minimising the exposure of healthy cells to the therapeutic agent. In this presentation, carbon nano-onions (CNOs) and carbon dots (CDs) will be discussed as a potential vesicles for nanocarrier-type drug delivery systems.[1] They are emerging as platforms for biomedical applications because of their ability to be internalized by cells and low toxicity both in vitro and in vivo. In my research group we synthesis and functionalize them. The carbon nanoparticles display high brightness and photostability in aqueous solutions and are selectively taken up by different cancer cell lines without significant cytotoxicity.

Silvia Giordani joined the School of Chemical Sciences at Dublin City University as Professor Chair of Nanomaterials in 2018 and took on the role of Head of School in 2020. Previously she received her PhD in Chemistry from the University of Miami, USA and carried out postdoctoral research at Trinity College Dublin (TCD) and at the University of Trieste, Italy. In 2007 she received the prestigious President of Ireland Young Researcher Award and was a Research Assistant Professor at TCD from 2007 to 2013. In 2013 she founded and directed the new “Nano Carbon Materials” research lab at the Istituto Italiano di Tecnologia (IIT) and in December 2016 she was appointed Associate Professor in Organic Chemistry at the University of Turin, Italy. Her main research interests are in the design, synthesis, and characterization of a wide range of nanomaterials for applications in smart and responsive bio-related nanotechnologies. She is the author/co-author of more than 140 manuscripts, reviews and book chapters. She is the recipient of many international prizes and honours including the L’Oreal UNESCO for Women in Science fellowship, the William Evans visiting fellowship from the University of Otago (New Zealand) and is a Visiting Scientist to the Bio-Nano Institute at Toyo University (Japan). Her research has been recently featured in “Where I work” published in Nature on the 20th May 2021.
- 11:00 - 11:30 Coffee break.
- 11:30 - 13:30. Session IV. Chair: Irina Milisav
NRF2 degradation during inflammation is coordinated by p38 γ/δ and GSK3 kinases.
Ana I Rojo1, Marta Pajares1, David Chamoso1, José Jiménez Villegas1, Ester Díaz Mora2, Ana Cuenda2, John D Hayes3, Albena Dinkova-Kostova3 and Antonio Cuadrado1.
1Department of Biochemistry, Faculty of Medicine, Autonomous University of Madrid and Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED), Spain. 2 Biotechnology National Center, Spain. 3 School of Medicine, University of Dundee, Scotland.
Chronic inflammation is common to many non-communicable diseases. NRF2 transcription factor has recently emerged as a key immunomodulator but the mechanisms governing its function are not well characterized. In this study, we approached this issue in LPS-stimulated macrophages. In the short term, LPS induced the phosphorylation of NRF2 while after several hours it led to a decrease in phosphorylation, together with protein stabilization, and finally a return to basal levels. In search for kinases that might be involved in this process we found that both, in vitro and in vivo NRF2 is phosphorylated by p38γ and p38δ at several serines. Interestingly, their overexpression led to an increase of NRF2 half-life supporting that both are involved in NRF2 activation. However, we found that Ser342 and Ser347, which are very close to the sequence phosphorylated by GSK3 and most likely participate in NRF2 destabilization, were also targeted by p38γ/δ. Analysis of the Neh6 domain by bidimensional gel electrophoresis evidenced that the phosphorylation exerted by GSK3 in Ser335 and Ser338 was impaired in Neh6 mutant versions (Ser342 and Ser347 to Ala) suggesting that p38γ/δ could be priming kinases for GSK3 to promote NRF2 degradation. In fact, sequential kinase assay revealed that previous phosphorylation of Neh6 by p38γ/δ actually improves phosphorylation of Ser338 by GSK3. Altogether, our data indicates that p38γ/δ participates in the modulation of NRF2 in a dual fashion: on one side contributing to NRF2 activation but on the other side priming the Neh6 domain for later degradation in cooperation with GSK3. This study allows a better understanding of the mechanistic regulation of NRF2 in diseases where inflammation is an important pathomechanism.

Antonio Cuadrado, is full professor of Biochemistry and Molecular Biology at the Department of Biochemistry, Medical School, Autonomous University of Madrid. He obtained his PhD degree in Biology in 1985 and enjoyed several postdoctoral stays in the National Cancer Institute-NIH with the help of Fulbright and Fogarty fellowships. He established his independent laboratory as Professor of Biochemistry in 1997 with a main interest on the study of molecular mechanisms involved in initiation and progression of chronic diseases. For the past years his main lane of research has been the validation of transcription factor NRF2, master regulator of cellular homeostasis as a new therapeutic target in chronic diseases with particular emphasis in neurodegenerative diseases (Alzheimer and Parkinson) and in fatty liver diseases. His current interest is the development of new NRF2-modulating drugs. Dr. Cuadrado has published over 160 primary and review articles, of which more than 80 are related to the role of NRF2 in physiological and pathological responses to disease.
Molecular mechanism of lipid accumulation in non-alcoholic fatty liver disease: new targets for the clinic
Nesrin Kartal Ozer
Department of Biochemistry, Faculty of Medicine, Uskudar University, Istanbul, Turkey.
email: nesrin.kartalozer@uskudar.edu.tr
The global prevalence of nonalcoholic fatty liver disease (NAFLD), which is characterized by excessive accumulation of fat in hepatocytes, is constantly increasing in parallel with the obesity syndrome. Recent studies have shown the abnormalities in LD biology and LD related proteins as an important factor in fatty liver disease development. Hence, understanding role of LD biogenesis proteins in lipid homeostasis may give alternate views on disease pathogenesis and treatment approaches. However high cholesterol is known to affect LD size, membrane structure, and function of membrane-associated protein. Additionally, despite the studies proposing the crucial role of high cholesterol in the development of fatty liver through hepatocyte lipid droplet biogenesis, its mechanism is largely unknown. Nrf2 plays a role in the regulation of signals such as oxidative stress and inflammation, which are important in the pathogenesis of many metabolic syndromes. However, although Nrf2 is known to regulate genes associated with lipid metabolism in hepatic steatosis, how Nrf2 signaling is affected in cholesterol-mediated LD accumulation is not yet clear. The aim of this study is to create a cholesterol-induced lipid accumulation model and to determine the effect of LD biogenesis on Nrf2 signaling. In this direction, LD number/size analysis was performed with Bodipy staining, while genes associated with Nrf-2 activation were evaluated with q-RT PCR. Results indicate molecular mechanism of lipid accumulation in NAFLD and role of Nrf-2 will be discussed.

Nesrin Kartal Ozer, is Professor at Marmara University, Faculty of Medicine, Department of Biochemistry, Istanbul,Turkey. She graduated with a BSc in Pharmacy from Hacettepe University, Ankara, Turkey and she has received her PhD in Biochemistry in Hacettepe University, Faculty of Medicine. She moved then to Marmara University, Istanbul. She was a Visiting Scientist at St.George’s Hospital, Medical School, Department of Biochemistry, London, UK (1985-1986); Institute of Biochemistry and Molecular Biology, University of Bern, Switzerland (1993-2004) and University of Hohenheim, Stuttgart, Germany (2008).
In her research she published ~ 100 articles and she was invited speaker at ~80 international meetings. She was the organizer ~10 scientific meetings and conferences.
As member of International Organizations, it is worth mentioning her membership in the International Advisory Committee, Oxygen Club of California (1999- today); FEBS Advanced Course Committee Member (2003-2006); FEBS Fellowships Committee Member (2021-2024), Secretary General Society for Free Radical Research-Europe (SFRR-E) and Member of the Council (2003-2009) and President of SFRR-E (2013-2014).
She was awarded The Oxygen Club of California Award and “Life Time Honorary Member” in 2006 “In recognition of outstanding research contributions on the role of vitamin E in atherosclerosis and to fostering the field of free radical biology” and The Society for Free Radical Research-Europe Lifetime Achievement Award in 2019 “In recognition of Lifetime Extraordinary Scientific Achievements in the Field of Free Radical Research”.
Her research focus on endoplasmic reticulum stress, redox signaling and cell death in metabolism related diseases such as cardiovascular diseases and non-alcoholic fatty liver disease. In parallel she has carried out research on the molecular function of tocopherols in these diseases.
ND-13, a DJ-1 derived peptide, as a novel pharmacological approach to regulate Nrf2 in the prevention of Diabetic Nephropathy
Santiago Cuevas Gonzales, Spain
BioMedical Research Institute of Murcia (IMIB-Arrixaca), Murcia (Spain)
email: Santiago.cuevas@imib.es
Diabetic nephropathy (DN) is the most important cause of renal failure worldwide and is characterized by sustained inflammation regulated in part by NLRP3 Inflammasome, thus, attenuating inflammation is a major priority to prevent renal damage. Our previous publications show that DJ-1 has antioxidant and anti-inflammatory properties in the kidney and regulates the master antioxidant switch Nrf2. ND-13 is a short peptide consisting of 13 amino acids of the DJ-1-protein, which could increase Nrf2/DJ-1 pathway. We demonstrated that ND-13 prevented renal fibrosis, inflammation, and renal injury in mice with renal damage. Our preliminary data show that ND-13 and Nrf2 prevents inflammasome activation in mouse macrophages. This presentation will present the most significant data published in this project and update the new data obtained to determine the role of DJ-1/Nrf2 pathway on inflammasome regulation, and their role to attenuate renal dysfunction in diabetes. Human peripheral blood mononuclear cells (PBMCs) from patients with DN and controls will be isolated from blood, cultured, and treated to determine the effect of the experimental treatment to attenuate inflammasome pathways activation. In addition, the data about the protective effects of ND-13 and DJ-1/Nrf2 pathway in DN induced by streptozotocin injection in C57Bl/6 and NLPR3-/- mice will be also included. Nrf2/DJ-1 may be a promising new target to attenuate oxidative stress and inflammation which are considered key factors in the pathogenesis of DN, and other renal diseases.

Santiago Cuevas. 22 years of experience in the academy and industry in basic, translational and clinical research studying the pathways involved in the regulation of oxidative stress and inflammation in the pathogenesis of hypertension, cardiovascular and renal diseases. Ten years of research experience in the United States on the field. At the present, I am a Principal Investigator Miguel Servet in the Institute of Biomedical Research in Murcia (IMIB). I am a team leader in the Unit of Molecular Inflammation at the IMIB led by Dr. Pablo Pelegrin, where we study the molecular mechanism involved in inflammasome regulation and its role in the pathogenesis of several diseases.
Increased DNA methylation of genes of proteasomal system in patients with type 1 diabetes and diabetic kidney disease
Natalia Paramonova1, Leonora Pahirko2, Emīls Siliņš2, Kristīne Baumane3,4, Līga Zariņa3, Jeļizaveta Sokolovska4
1University of Latvia, Institute of Biology, Riga, LV1004, Latvia
2University of Latvia, Faculty of Physics, Mathematics and Optometry, Riga, Latvia
3Riga East University hospital, Ophthalmology department, Riga, Latvia
4University of Latvia, Faculty of Medicine, Riga, Latvia
Email: jelizaveta.sokolovska@lu.lv
Objective. Among different molecular mechanisms of diabetic kidney disease (DKD), impaired protein degradation and hypoxia-driven processes are of interest. DNA methylation of genes of ubiquitin-proteasome system and HIF1A have not been described in DKD yet. The aim of this study was to investigate the level of DNA methylation in the promoter regions of PSMA6, PSMB5 and HIF1A genes in patients with different severity of DKD and type 1 diabetes (T1D).
Design. This was a cross-sectional study, 114 T1D patients with different DKD stages were analyzed. Bisulfite-free, restriction enzyme-dependent determination of the DNA methylation status in the promoter regions of the genes PSMA6, PSMB5 and HIF1A was performed using real-time PCR and OneStep qMethyl™ Kit (Zymo research). DNA methylation extent was calculated using threshold cycle or CT values and the following equation: 100*2(-ΔCт).
Results. The highest levels of DNA methylation in the investigated genes were observed in advanced DKD, but only for PSMB5 gene we observed statistically significant difference in DNA methylation between DKD groups (no DKD 2.3 (1.3-3.4), initial DKD 2.9 (1.8-4.8), advanced DKD 3.8 (2.8-14.7), p<0.001). Methylation profile of all investigated genes, correlated positively significantly with diabetes duration (HIF1A r=0.309, p<0.001; PSMA6 r=0.295, p=0.002; PSMB5 r=0.282, p=0.002) and between each other.
Conclusion. DNA methylation of PSMB5 correlates with severity of DKD.

Jelizaveta Sokolovska, MD, PhD, internist, endocrinologist is a practicing clinician and leading researcher at the University of Latvia. She has scientific expertise in the field of diabetes, complications of diabetes, endocrinology, clinical research, nutrition. Current scientific directions include biomarkers in diabetic retinopathy and nephropathy; intestinal inflammation and microbiome changes as a potentially modifiable risk factor for complications in type 1 diabetes; application of artificial intelligence methods for patient stratification in monitoring and prevention of diabetic complications; lifestyle and diet for prevention of diabetic complications.
Klotho-Nrf2 interaction ameliorates acute kidney injury and long-term outcomes associated with rhabdomyolysis.
Melania Guerrero-Hue1, Mercedes Vallejo-Mudarra1, Cristina García-Caballero1, Lucía Beltrán-Camacho1,2, José Luis Morgado-Pascual1,2, Alfonso Rubio-Navarro 3,4, Juan Antonio Moreno1,2.
1. Maimonides Biomedical Research Institute of Cordoba (IMIBIC), Hospital Universitario Reina Sofía, 14004 Córdoba, Spain.
2. Department of Cell Biology, Physiology and Immunology, University of Cordoba, 14014 Cordoba, Spain.
3. Instituto de Investigación Biosanitaria de Granada (ibs.GRANADA), 18012 Granada, Spain.
4. Excellence Research Unit “Modeling Nature” (MNat), University of Granada, 18016 Granada, Spain.
Email: juan.moreno@imibic.org
Rhabdomyolysis is a serious clinical syndrome caused by skeletal muscle damage that may lead to acute kidney injury (AKI) and chronic loss of renal function. Klotho is an anti-aging protein that is mainly expressed in the kidney. Klotho protects against oxidative stress, inflammation and fibrosis, commons outcomes of rhabdomyolysis-AKI. However, the role of Klotho in rhabdomyolysis remains unexplored. We found decreased serum and renal Klotho levels in patients and mice with rhabdomyolysis. Klotho downregulation was related with acute loss of renal function, kidney injury, and inflammation at early stages of rhabdomyolysis. Klotho expression remained reduced one month after rhabdomyolysis-induction, when renal function returned to basal, but kidneys showed features of chronic damage, such as fibrosis and inflammation. Pathway analysis ranked Nrf2 as the top predicted transcription factor regulating expression of Klotho. STAMP software also identified similarities between the sequence of the promoter region of Klotho and the consensus sequences of Nrf2 antioxidant response elements (ARE). Nrf2 inducers sulforaphane and curcumin increased Klotho expression and augmented the expression of HO-1, a protein transcriptionally regulated by Nrf2. Next, we observed that Nrf2 inducers prevented Mb-related Klotho downregulation. To evaluate the beneficial effects of Nrf2 activation in vivo, we treated mice with curcumin in two different therapeutic approaches. First, treating mice before glycerol injection to evaluate a potential preventive effect and second, treating mice after rhabdomyolysis induction to assess the effect of curcumin as curative treatment. Both strategies improved renal function and prevented rhabdomyolysis-mediated Klotho downregulation. Furthermore, genetic deletion of Nrf2 in mice promoted a dramatically reduction in Klotho mRNA and protein expression in comparison with wild-type mice. In conclusion, Klotho levels are reduced not only at early stages of rhabdomyolysis-AKI, but also at later phases, when adverse long-term consequences are reported. Nrf2-based strategies to recover or maintain Klotho levels may represent potential therapies to decrease acute consequences of rhabdomyolysis-AKI and to prevent their chronic negative effects.

Juan Antonio Moreno is a Ramon y Cajal Tenure Track Researcher at the Department of Cell Biology, Physiology and Immunology (Cordoba University, Spain). Head of the Group GE-06 “Pathophysiology of renal and vascular damage” at Maimonides Research Institute of Cordoba (IMIBIC). My group is unravelling novel pathogenic mechanisms involved in the development of renal and vascular diseases (atherothrombosis, diabetic nephropathy, glomerular diseases, acute kidney injury, renal fibrosis, among others). We aim to understand the basis of the alterations occurring in both kidney and arterial wall by targeting specific pathways to decrease the progression of these pathologies. Specifically, we are interested in certain cellular and molecular mechanisms, such as oxidation, inflammation, apoptosis and fibrosis. In the last years, we have evaluated the role of Nrf2 in acute kidney injury and are interested in the validation of novel compounds targeting Nrf2 to decrease both renal and vascular damage.
Regulation of cellular inflammatory processes be extracellular vesicles
Virginijus Tunaitis
Department of Stem Cell Biology, State Research Institute Centre for Innovative Medicine, LT-01102, Vilnius, Lithuania.
Email: virginijus.tunaitis@imcentras.lt
Extracellular vesicles (EVs) are heterogeneous group of membrane coated structures secreted by cells into their surroundings and dedicated for intercellular communication. EVs includes three main groups of vesicles – exosomes, ectosomes and apoptotic bodies. EVs are found in all body fluids and can be selectively taken up by target cells located in the different parts of organism. Intercellular communication includes delivery of lipids, proteins, nucleic acids from cell to cell and are able to maintain homeostasis, suppress or induce inflammation. In our early experiments we found that exosomes secreted by human exfoliated deciduous teeth stem cells (SHED) suppress carrageenan-induced acute inflammation in mice. The same anti-inflammatory effect of EVs was observed in experiments using Parkinson disease (PD) model. We found that EVs derived from MSC-like cells are able to modulate neuro-inflammatory processes in vitro and in vivo. Data reveal that EVs, particularly exosomes, suppress 6-hydroxydopamine (6-OHDA)–induced apoptosis in dopaminergic neurons in vitro. In vivo experiments in a rat model demonstrate the therapeutic efficacy of intranasal administration of EVs derived from SHEDs. We found that EVs can effectively suppress 6-OHDA-induced gait impairments and normalize tyrosine hydroxylase expression in the striatum and in the substantia nigra of the 6-hydroxydopamine-treated rats. Data suggest that EVs induce neuroprotective actions by simultaneous targeting of dopaminergic neurons and by possible modulation of astroglial and microglial responses to the injury of nigra-striatal pathways. Although the mechanism of neuroprotective action of EVs remains largely unexplored, some evidence point to the importance of TLR4 and Nrf2 signaling.

Virginijus Tunaitis is a Senior research associate at the Department of Stem Cell Biology, Centre for Innovative Medicine, Vilnius. His research is focused on the human mesenchymal stem cells (MSCs)-derived extracellular vesicles and neurodegenerative disease modeling in vitro.
- 13:30 - 14:30 Lunch
- 14:30 - 16:00. Session V. Chair: Lidija Milković
Protein-protein interactions in the regulation of NRF2 signaling
Anna-Liisa Levonen1
1A.I.Virtanen Insitute for Molecular Sciences, Faculty of Health Sciences, University of Eastern Finland, Finland
Email: anna-liisa.levonen@uef.fi
The Kelch-like ECH-associated protein 1 (KEAP1) – Nuclear factor erythroid 2 -related factor 2 (NRF2) pathway is the major transcriptional response system in cells protecting against oxidative and electrophile stress. While the key proteins of the pathway and their role in signaling are well known, the complex interactions with other proteins that affect the signaling processes have not been thoroughly studied. Moreover, NRF2 is frequently constitutively active in many cancers due to somatic loss-of-function mutations in KEAP1 or gain-of-function mutations in NRF2 hindering KEAP1-NRF2 protein-protein interaction (PPI), yet how these mutations might alter other protein interactions is not known. The objective of this study is therefore to study using systems proteomics approaches how PPIs of KEAP1 and NRF2 are altered by mutations common in non-small cell lung cancer. To this end, we used affinity purification and mass spectrometry (AP-MS) analysis of wild type KEAP1 and the mutants R320Q and R470C to interrogate differences in the protein interactome. To capture transient interactions of NRF2 protein, proximity-dependent biotin identification (BioID) was used to elucidate the interactions of wild type vs. D29N and E79Q mutations within KEAP1 interacting DLG- and ETGE-motifs. Interestingly, both KEAP1 mutant forms showed an enrichment of proteins involved in TNFα signaling, indicating crosstalk of the two pathways. With respect to NRF2, we identified known interactors such as KEAP1, MAFG and PGAM5 but also many other proteins previously not known to interact with NRF2. We also discovered that NRF2 mutants bind to nuclear proteins more avidly than wild type NRF2, indicating enhanced chromatin occupancy. Among the identified proteins, a number of chromatin remodeling proteins, histone modifiers as well as transcriptional coregulators were found, likely impacting NRF2-dependent signaling.

Anna-Liisa Levonen is currently Professor and Vice Dean of Research at the University of Eastern Finland, Faculty of Health Sciences. She is also Associate Editor of Redox Biology. She earned her MD and PhD degrees from the University of Helsinki, Finland in 1994 and 2000, respectively, followed by a fellowship at the University of Alabama at Birmingham (UAB), Department of Pathology and Center for Free Radical Biology. Upon her return to Finland, she was recruited to the University of Kuopio (currently University of Eastern Finland), where she has established a research program focusing on the gene regulatory mechanisms activated by oxidative and electrophilic stress, particularly via the redox-activated transcription factor NRF2. She has studied the mechanism of activation of the KEAP1-NRF2 pathway and its role in disease, particularly cancer and cardiometabolic diseases, and has published highly cited original and review articles on the topic.
disorders.
Uncovering the Gender-Specific Effects of Toxic Metal(oid) Mixtures on NRF2 and HO-1 in Rat Liver and Kidneys
Dragana Javorac, Katarina Baralic, Djurdjica Maric, Evica Antonijevic Miljakovic, Marijana Curcic, Danijela Djukic-Cosic, Biljana Antonijevic, Aleksandra Buha Djordjevic
Department of Toxicology, University of Belgrade-Faculty of Pharmacy, Belgrade, Serbia
Email: aleksandra@pharmacy.bg.ac.rs
Toxicological research focuses on the toxicity of individual metals, which does not reflect the real-world environment. Based on the human biomonitoring study, six toxic metal(oid)s (As, Pb, Hg, Cd, Cr (VI), Ni) were chosen and their effect on NRF2 signaling in the liver and kidneys of rats was investigated. The aim of the study was to investigate the effect of a real-life toxic metal(oid) mixture on the levels of NRF2 and heme-oxygenase (HO-1) in rats after 90 days of exposure to different doses of this mixture. Animals were given toxic metal mixture solutions in three different doses that corresponded to doses calculated based on the results of a human biomonitoring study (M1, F1: median dose; M2, F2: 95th percentile exposure; M3, F3: Benchmark doses). NRF2 and HO-1 levels were determined in liver and kidney samples using Rat Nfe2l2 ELISA Kit (Wuhan Fine Biotech Co., Wuhan, China) и Rat HO-1 ELISA Kit (Wuhan Fine Biotech Co., Wuhan, China). An ANOVA test was used to analyze the results, followed by an LSD test. In both sexes’ livers and female kidneys, a statistically significant decrease in NRF2 was obtained in the M1, M2, M3, and F3 groups, whereas in male kidneys, a statistically significant increase in NRF2 was observed in the M3 group. Female livers and male kidneys had statistically significant decreases in HO-1 in the F3, M2, and M3 groups, while male livers in the M3 group had a statistically significant increase in HO-1. In female kidneys, no statistical significance was found.
The study’s findings show that under certain conditions, depending on gender and organ, toxic metal(oid) mixtures have different toxic effects on NRF2 and HO-1 and these effects can contribute to their toxic effects in liver and kidneys.

Aleksandra Buha Djordjevic is an associate professor of Toxicology at the Department of Toxicology, University of Belgrade – Faculty of Pharmacy. Her main research interest is the toxicology of mixtures and endocrine-disrupting chemicals. In the field of NRF2 signaling, her special interest is in the role of this pathway in the toxicity of environmental toxicants. She is the author/co-author of more than 80 peer-reviewed publications, her h index is 26 and her work has been cited more than 2000 times according to SCOPUS. Dr. Buha Djordjevic has been included in the Stanford University list of top 2% scientists in the world for 2020 and 2023. She is a MC member and science communication coordinator COST action CA 20121 „Bench to bedside transaction for pharmacological regulation of NRF2 in noncommunicable diseases“.
Mcc1 binds Keap1 to regulate Nrf2 expression in the Th17/Treg axis in inflammatory bowel disease
Norwin Kubick1, Irmina Bieńkowska2, Jarosław Olav Horbańczuk3, Mariusz Sacharczuk2, Michel Edwar Mickael2,4,*
1University of Hamburg, Department of Biology, Institute of Plant Sciences and Microbiology and biotechnology, Ohnhorststr. 18, 22609 Hamburg
2Department of Experimental Genomics, Institute of Animal Biotechnology and Genetics, Polish Academy of Science, Postępu 36A, 05-552, Poland.
3 Institute of Animal Biotechnology and Genetics, Polish Academy of Science, Postępu 36A, 05-552, Poland.
4 PM Research Center, Väpnaregatan 22, 58649 Linköping, Sweden
Email: m.mickael@igbzpan.pl
In peripheral immunity diseases such as inflammatory bowel disease (IBD), naïve CD4+ T cells differentiate into pathogenic Th17 (Th1)-like T cells. During this process, the possibility of bifurcation toward less inflammatory types such as typical Th17, RORγt+Treg, or FoxP3 Treg becomes diminished. Once pathogenic Th17 mature, they increase the inflammation profile of the gut, exacerbating the diseases as well as specifically infiltrating the blood-brain barrier causing localized brain inflammation. On the other hand a significantly smaller number of naïve CD4+ T cells have the ability to differentiate toward an anti-inflammatory phenotypes such as FoxP3+ Tregs. We have shown that adoptive transfer of FoxP3+ Tregs to the gut at the initial stages of inflammation prevent progression of the diseases and inhibit Th17(Th1) infiltration of the brain. Thus, identifying the mechanisms of differentiation of anti-inflammatory phenotypes along the Th17/Treg axis is crucial for finding novel therapeutic strategies to treat for IBD. We analyzed both bulk RNA-seq datasets as well as scRNA-seq focusing on comparing Th0 progression into Th17 and Tregs to pin point potential molecular networks that are responsible for the activation of the anti-inflammatory axis. Using network rewiring techniques on genetic and gene enrichment levels allowed us to identify genes and pathways that were alerted between the Th0/Th17 axis and Th0/Treg axis as well as between Th17 and Tregs. Our analysis identified the gene Mcc1 with fold change large than 2 and adjusted P-value <0.003 to be upregulated in Tregs in comparison with Th17 and Th0. Our investigation, based on MCC1 structural similarity, suggests that they Tregs’ differentiation through regulating the oxidative stress pathways. Specifically, we found that the MCC1 motif PSTGE binds KEAP1 with high affinity to compete with KEAP1 binding to NRF2 in order to regulate its expression. Taken together, our results highlights the importance of exploring anti-oxidative stress therapeutic strategies to cure inflammatory conditions such as IBD.
Michel is assistant professor focusing on immunology at the Institute of Genetics and Animal Biotechnology of the Polish Academy of Sciences (IGHZ) at the department of experimental genomics. Prior to starting his tenure as an assistant professor, Michel was trained as a postdoc at Uppsala University, Sweden; UKE, Hamburg, Germany; and the Victor Babes Institute, Romania. He worked as a visiting scientist at UAB in Alabama. Prior to that, he earned his PhD from Durham University. Michel research is dedicated to understanding the role of the adaptive immune system in neuroinflammatory diseases on a single-cell level. One aspect of his research focuses on the gut-brain axis during colorectal cancer and colitis. In particular, Michel is interested in studying the CD4+ Th17/Treg network and its role in linking microbiota, dietary components, and brain inflammation during colitis and colorectal cancer. To study this interesting biological landscape, various immunological assays are employed, including sorting of CD4+ T cell subtypes using flow cytometry, in vitro assays (differentiation and co-culture), and in vivo assays (adoptive transfer). Michel’s interests span both basic immunology animal research and translational human-based research. Michel is also an expert in using cutting-edge computational methods such as next-generation sequencing, including RNA-seq, CHIP-seq, and single-cell RNA-seq (10x and Dropseq), as well as AI mining of TCR-microbiota species data. An important aspect of Michel’s work is teaching various academic courses. Furthermore, Michel is supported by various national and international grants.
Activation of NRF2 by environmental stressors provides an opportunity for fast screening reporter assays for evaluation of biological toxicity
Ivana Kuntic1, Marin Kuntic1, Hartmut Kleinert2, Andreas Daiber1,3
1 Department of Cardiology 1, University Medical Center of the JGU, Mainz, Germany
2 Department of Pharmacology, University Medical Center of the JGU, Mainz, Germany
3 German Center for Cardiovascular Research (DZHK), Partner Rhine-Main (Mainz), Germany
Email: daiber@uni-mainz.de
Environmental risk factors, including noise, air pollution, chemical agents, ultraviolet radiation (UVR) and mental stress have a considerable impact on human health. Oxidative stress and inflammation are key players in molecular pathomechanisms of environmental pollution and risk factors. Environmental pollutants are known to activate the nuclear factor erythroid 2-related factor 2 (NRF2), e.g. by induction of oxidative stress and inflammation. We here discuss the potential use of a heme oxygenase-1 promoter (HO1prom) activity assay as a read-out of NRF2 activation by environmental air pollution (particulate matter, PM). Ambient PM (NIST particles, SRM 1648a) caused a dose-dependent increase in the luciferase chemiluminescence signal in the HO1prom reporter cells. Actually, we test the impact of size distribution of particles using nano- to micrometer scale carbon black particles. In order to prove the HO-1 induction via NRF2 we test NRF2 inhibitors. In order to investigate whether particles induce oxidative stress to activate NRF2 and induce HO-1 we test direct scavengers of reactive oxygen species (ROS), namely TEMPO, PEG-SOD and PEG-catalase. For discrimination between direct ROS formation by particles and activation of cellular ROS sources we test NADPH oxidase inhibitors GSK2795039 and VAS2870 and mitochondrial ROS scavenger mitoTEMPO. HO-1 induction will be correlated with cell viability for evaluation of biological toxicity of the stressors. After full characterization of the mechanism of HO-1 induction, we will test other environmental toxins.

Andreas Daiber studied Chemistry at the University of Konstanz (Diploma in 1997), holds a PhD in Biochemistry (graduated in 2000), did his habilitation (graduated in 2006), and since 2008 is a full professor in Molecular Cardiology at the University Medical Center Mainz. >33 significant research grants from the pharmaceutical industry and public funding bodies. 2011 guest professorship at the Université Joseph Fourier at Grenoble, France. From 2014-2016 Chair of COST Action BM1203 (EU-ROS). Memberships in national and international scientific communities (SFRBM/SFRRE, ASBMB, DGK), reviewer activities for numerous scientific journals (e.g. FRBM, Redox Biology, ATVB, Eur. Heart J., Nat. Comm.) and funding bodies, editorial board positions (Oxid. Med. Cell. Longev., Cardiovasc. Res., Antioxidants, FRBM, Redox Biology), guest editor (Antioxid. Redox Signal., Redox Biology, Br. J. Pharmacol., FRBM, Antioxidants). He published >195 original research articles, >150 review articles, 26 book chapters, >180 conference abstracts and 3 patents with Boehringer Ingelheim. h-index: 75; >16,600 citations. Special research interests: redox biochemistry, oxidative stress and environmental research in cardiovascular disease.
- 15:45 - 16:20. Coffee break
- 16:20 - 17:30 | Poster session.
Effect of DMF treatment in crowding stress-exposed prehypertensive rats
Iveta Bernatova1, Andrea Micurova1, Michal Kluknavsky1
1 Centre of Experimental Medicine, Slovak Academy of Sciences, Institute of Normal and Pathological Physiology, Bratislava, Slovakia,
Email: Iveta.Bernatova@savba.sk
Despite the accelerating information on the roles of nuclear factor erythroid 2-related factor 2 (NRF2, encoded by Nfe2l2 gene), less is known about the NRF2 signalling in conditions of chronic social stress in rats with a genetic predisposition to hypertension. We investigated changes in the expression of Nfe2l2 and several NRF2 target genes in the liver and heart of adult male borderline hypertensive rats (BHRs) in conditions of chronic social stress induced by crowding. In addition, the hypothesis was tested that the administration of NRF2 activator dimethyl fumarate (DMF) can prevent the development of stress-induced hypertension in BHRs. BHRs were exposed to crowding for 6 weeks with or without oral DMF (30 mg/kg/day p.o.) treatment. Crowding resulted in elevated blood pressure and reduced body weight gain vs. controls. DMF completely prevented stress-induced BP increase and partially improved body weight gain. DMF reduced relative liver weight but had no effect on heart weight when considered as the main effect of treatment in 2-way (stress x DMF treatment) ANOVA analysis. The levels of conjugated dienes were reduced by stress in both tissues investigated (main effect of stress). In the liver, DMF elevated CD levels, on the other hand, in the heart DMF reduced CD (main effects of DMF). Gene expressions of Nfe2l2, Pparg and Ppara, were all significantly elevated by stress in the liver. DMF reduced Nfe2l2 expression in the liver however no changes were found in expressions of Hmox1, Sod1 and Gpx4. In contrast, in the heart Nfel2l2 expression was elevated by both stress and DMF (main effects) without changes in Pparg and Ppara expressions. All, Hmox1, Sod1 and Gpx4 expressions were significantly elevated in the heart of DMF-treated rats as well as in rats exposed to DMF+ stress vs. controls. In conclusion, our data showed that DMF prevented the development of stress-induced hypertension in rats with a genetic predisposition to hypertension. However, different effects of DMF on Nf2l2 and antioxidant enzymes genes Hmox1, Sod1 and Gpx4 were found in the heart and liver.

Iveta Bernatova is a senior scientist, Head of the Department of Experimental Hypertension and a Member of the Management Board of Centre of Experimental Medicine, v.v.i., Slovak Academy of Sciences, Bratislava, Slovakia. She studied Biochemistry (Diploma in 1991), holds PhD in Chemistry (1997) and a title Doctor of Science (D.Sc.) in Animal Physiology (2009). Her research is focused on the regulatory mechanisms of blood pressure in various experimental models of hypertension and the ways of prevention and treatment of high blood pressure with particular attention paid to the role of nitric oxide and oxidative stress in the regulation of blood pressure and vascular function. A significant part of her research is focused on the role of chronic social stress in the development of hypertension and on mechanisms of adaptation to stress. The most recent studies are focused on the research of iron metabolism in the heart and liver, the role of NRF2 in the regulation of iron metabolism in rats with various genetic predispositions to hypertension as well as on the effect of acute and chronic stress in NRF2-mediated mechanisms. She is the author of 120 peer-reviewed publications with around 2000 citations.
The study was supported by project VEGA No. 2/0157/21.
Identification of blood-based miRNAs regulating redox and inflammatory genes in Alzheimer Disease
Elena Milanesi1,2, Maria Dobre1, and Gina Manda1.
1 “Victor Babeș” National Institute of Pathology, Bucharest, Romania
2 Faculty of Medicine, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
Email: elena.k.milanesi@gmail.com; elena.milanesi@ivb.ro
Biological aging is associated with a chronic background of inter-related low-grade inflammation and oxidative stress (“inflammaging”) that is believed to be a significant risk factor for the acceleration of the aging process and the worsening of various age-related diseases, including Alzheimer Disease (AD). These disturbances appear long before symptoms onset and accompany disease evolution. In a previous study based on gene expression pattern in blood, followed by Receiver Operating Characteristic analysis, we identified nine inflammation and seven redox upregulated genes that discriminated well between AD patients and controls (area under the curve >0.9). Two well-known NRF2 target genes, GSR and GSTP1, were found among the top significantly upregulated genes. GSR overexpression was evidenced also in the blood of APPV717I × TAUP301L mice which partially recapitulate with age the amyloidopathy and tauopathy of the human disease.
In this study, in the whole blood of 24 AD patients and 24 age-matched controls, enrolled in the previous study, we identified through miRNet 2.0 analysis, and further measured the expression of six miRNAs targeting the above-mentioned 17 upregulated inflammation and redox-related transcripts. Four miRNAs (miR-15b-5p, miR-16-5p, miR-24-3p, and miR-146b-5p) were significantly downregulated (p<0.05, Fold Regulation <-1.5) in AD patients. Significant negative correlations were found between GSR and GSTP1, and the following two miRNAs: miR-16-5p (p=0.007, r=-0.386; p=0.001, r=-0.468, respectively) and miR-146b-5p ((p=0.012, r=-0.362; p=0.001, r=-0.460, respectively). These results further confirm the involvement at systemic level of the NRF2 signaling pathway along with inflammation in the AD pathogenesis. ACKNOWLEDGEMENT: This work was funded by the national fellowship program L’Oreal – Unesco “For Women in Science”.

Elena Milanesi is Assistant Professor at the Faculty of Medicine Carol Davila of Bucharest and researcher at the National Institute of Pathology Victor Babes, Bucharest, Romania. She studied Medical Biotechnologies at the University of Brescia Italy, where she holds a PhD in Molecular Genetics Applied to Medical Sciences (2012). She worked two years at the Sackler Faculty of Medicine of Tel Aviv, Israel as Post-Doc. Currently, she is Principal Investigator of the ICRP/ICGEB project “The brain-gut axis linking inflammatory bowel disease with anxiety and depression: the inflammation-microbiome network” (2022-2024) and principal Investigator of the project “The regulatory effect of miRNAs on redox and inflammatory alterations identified in cognitive impairment” funded by the national fellowship program L’Oreal – Unesco “For Women in Science” (December 2022-2023). Since October 2021 she is Gender Equality Officer and from February 2023 GH Manager of the COST ACTION CA2012.
Effect of repurposed drugs on autofluorescent granule accumulation in a cellular model of age-related macular degeneration
Vicente L Miguel, Ana S Falcão, Margarida Pedro, Tomás Guerreiro, Miguel Seabra and Sandra Tenreiro
iNOVA4Health, NOVA Medical School|Faculdade de Ciências Médicas, NMS|FCM, Universidade Nova de Lisboa; 1169-056 Lisboa, Portugal
e-mail: vicente.miguel.ext@nms.unl.pt
Age-related macular degeneration (AMD) is one of the most common blinding illnesses in the developed world and its prevalence is expected to increase significantly in the next few decades as the world’s populations age. The retinal pigment epithelium (RPE) cells appear to accumulate autofluorescent lipofuscin from undigested photoreceptors outer segments (POS), leading to the formation of drusen deposits in the RPE-choroid interface and to the onset of the disease. To simulate this condition in vitro, we are using a monolayer of RPE cells in culture and pulsing it with a large amount of POS to induce the production of autofluorescence granules (AFGs) which are analogous to the lipofuscin-filled lysosomes and to subject the RPE to the same stress experienced by the diseased cells in vivo. Our previous results showed that the over-expression of the transcriptional regulator NRF2 can alleviate and aid in the processing of the AFGs. Thus, in this project, we are repurposing compounds that are already being tested for other pathologies such as Curcumin, Bardoxolone methyl, Sulforaphane and Dimethyl fumarate (DMF), which are known to affect NRF2 activity, to evaluate their effect on AFG formation in our in vitro model of AMD. We used DMF as a reference drug since in previous studies it significantly decreased AFG accumulation. Our preliminary data show that curcumin also can significantly decrease AFGs, pointing out this drug as very promising for the treatment of early and intermediate stages of AMD. This project was funded by “La Caixa Foundation” (NASCENT HR22-00569) and “New Talents’ Scholarship” from the Calouste Gulbenkian Foundation.

Vicente Miguel is a trainee working in Sandra Tenreiro’s DEGENERATION AND AGEING research group that focuses on clarifying the molecular mechanisms of retinal degeneration in the context of ageing diseases, using cellular models RPE and 3D retinal organoids, differentiated from human iPSc. VM is a 3rd-year bachelor’s student in biology with a specialization in genetics and molecular biology at Faculdade de Ciências da Universidade de Lisboa (FCUL) and has a particular interest in the fundamental and mechanistic study of Ageing. Vicente won the ‘New Talents’ scholarship from the Calouste Gulbenkian Foundation in late 2022 and has since worked in ST’s group.
NRF2/KEAP1 pathway alterations affect the progression of lung squamous cell carcinoma
Miriam Sánchez-Ortega1, Antonio Garrido1, Ana Clara Carrera1
1 Department of Immunology and Oncology, Centro Nacional de Biotecnología, Consejo Superior de Investigaciones Científicas (CSIC), Universidad Autónoma de Madrid, Cantoblanco, E-28049 Madrid, Spain.
Email: msanchez@cnb.csic.es and acarrera@cnb.csic.es
NRF2 is the master regulator of the antioxidant response, and its activity is mainly regulated by KEAP1. Several reports have described overactivation of this signaling pathway in several tumor types. However, less is known about its role in lung squamous cell carcinoma (LUSC), a tumor that does not have available any targeted therapies. Additionally, NFE2L2/KEAP1 is altered in ~30-40% of LUSC. Therefore, there is a need of understanding well the NRF2/KEAP1 action in LUSC.
In this work, the NRF2/KEAP1 pathway was found to be overactivated in LUSC samples harboring NFE2L2/KEAP1 alterations (as detected by increased NRF2 target gene expression). This provides active-NRF2 LUSC samples with an advantaged response against oxidative stress. Accordingly, NFE2L2 silencing impaired cell viability in vitro (under oxidative stress) and in vivo, in tumor xenografts, suggesting both the tumor driver nature of NRF2 in these tumors and the addiction to NRF2 in active-NRF2 LUSC. This aimed the study of potential genes acting downstream of NRF2 pathway responsible for tumor cell survival. This was studied using the CRISPR/Cas9 activation technology. The infection of a whole genome sgRNA library in the active NRF2 LUSC cells subjected to NRF2 depletion and to oxidative stress showed a lower proportion of cell death compared to cells without sgRNA library infection. Thus, the increased expression of some genes (HITs) by the library was able to trigger tumor cell survival and could be potential therapeutic targets. Further analyses and validation tests are needed to evaluate the importance of the obtained HITs as survival gene candidates involved in LUSC progression.

Miriam Sánchez studied Health Biology at the University of Alcalá (2016) and hold a PhD in Molecular Biosciences at the University Autonomous of Madrid (2023) under the supervision of Dra. Ana Clara Carrera and Dr. Antonio Garrido. During these years, she has been focusing on lung cancer, trying to find novel therapeutic targets for lung squamous cell carcinoma related to NRF2/KEAP1 pathway.
Aquaporins and Nrf2: Partners in Regulating Cellular Oxidative Stress
Monika Mlinarić1, Ana Čipak Gašparović1, Andrey Y. Abramov2
1Division of Molecular Medicine, Ruđer Bošković Institute, Croatia
2Department of Clinical and Movement and Neurosciences, UCL Institute of Neurology, UK
Email:Monika.Mlinaric@irb.hr
Aquaporins (AQPs) are membrane channels that facilitate the transport of water, glycerol, and other small solutes across the membrane in response to the osmotic gradient, and therefore play an important role in cell physiology. Peroxiporins, AQPs that transport hydrogen peroxide (H2O2), could play a role in the regulation of cellular oxidative status, consequently regulating important signaling pathways. Since they regulate H2O2 transport, there is a possible interaction between AQPs and Nrf2, the main regulator of antioxidant and cellular protective genes. We investigated AQP activity and their role in the regulation of oxidative stress by measuring the transport of H2O2 using fluorescent indicators Hyper-3 and OxiVision Green. Three non-selective AQP inhibitors (CuSO4, AgNO3, and NiCl2) were used to confirm the effect was due to AQPs, and Nrf2 was stimulated with Omaveloxolone to assess its potential effect on AQP activity. Cell death was evaluated with Hoechst and Propidium iodide staining, and reduced glutathione (GSH) levels in the cells were measured using the monochlorobimane (MCB) method to observe the effects of inhibiting AQP activity and stimulating Nrf2. Our results indicate that both inhibiting AQP activity and activating Nrf2 significantly reduced cell death. While treatment with AQP inhibitors showed some protective effect on GSH levels, Omaveloxolone was able to restore them to control levels. Furthermore, H2O2 influx to the cell was significantly reduced with both the inhibition of AQPs and activation of Nrf2, and the effect was amplified when both treatments were used together. These findings suggest that AQPs may play a critical role in regulating oxidative stress and that their activity may be modulated by Nrf2.

Monika Mlinarić is a PhD student working in the Laboratory for Oxidative Stress, Division of Molecular Medicine at Ruđer Bošković Institute. She is working on the project “Elucidating the role of aquaporins 3 and 5 in the development of breast cancer resistance to oxidative stress (AquaBCaRe)” with Dr. Lidija Milković and Dr. Ana Čipak Gašparović. As a topic of her PhD, she will investigate the interaction of Nrf2 and aquaporins (channels that facilitate the transport of hydrogen peroxide across the membrane), as well as their response to the state of chronic oxidative stress.
Exploring the link between AQP3, NRF2/KEAP1 signaling, and FOXO transcription factors in breast cancer cells under EGF stimulation and lipid raft disruption
Ivan Lucic, Monika Mlinaric, Ana Cipak Gasparovic, Lidija Milkovic
Laboratory for Oxidative Stress, Division of Molecular Medicine, Rudjer Boskovic Institute, Zagreb, Croatia
Email: Ivan.Lucic@irb.hr
Upon an increase in reactive oxygen species (ROS), nuclear factor-erythroid factor 2-related factor 2 (NRF2) is activated and enhances the expression of genes that protect cells from harmful ROS effects. However, ROS also play a vital role in normal cellular functioning by influencing various redox-sensitive signaling pathways. AQP3, a membrane pore commonly upregulated in breast tumors, facilitates water and small molecule transport and is a peroxiporin that enables the transport of hydrogen peroxide, the main ROS involved in redox signaling. Dysregulation of FOXO1 and FOXO3a, members of the Forkhead box O (FOXO) family of transcription factors, can contribute to the development and progression of breast cancer. The activation of EGFR receptors affects downstream signaling pathways that influence cancer cell survival.
This study aimed to investigate the impact of AQP3 on the NRF2/KEAP1 signaling pathway and the expression of FOXO transcription factors in different breast cancer cell lines under EGF stimulation and/or lipid raft disruption. The results indicate that AQP3 affects NRF2 and FOXO, suggesting a link between them that warrants further investigation. Depending on the presence or absence of AQP3, cell viability, and pathway activation were differently affected by lipid raft disruption and EGF treatment in the three breast cancer cell lines.

Ivan Lučić is a PhD student working in the Laboratory for Oxidative Stress, Division of Molecular Medicine at Ruđer Bošković Institute. Although his background is limited as he just started working, he has been actively involved in several research projects. His master’s thesis focused on the interaction between aquaporin 3 and the EGFR/PI3K/AKT signaling pathway in breast cancer cell lines. For his PhD research, he will further investigate the relationship between breast cancer, oxidative stress, and NRF2.
- 17:30 - 18:00 | Miscellaneous. Concluding remarks
- 20:30. Gala dinner
















