October 13th
- 9:30 - 10:00 Registration.
- 10:00- 13:00. Management MeetingChair: Prof. Antonio Cuadrado, Spain. Only for MC members
- 13:00 - 14:00 Lunch
- 14.00-16:00. Session I. Chair: Manuela G López
The exposome and ageing: targeting Nrf2
Professor Paul Shiels
Glasgow Ageing Research Network (GARNER), School of Molecular Sciences, MVLS, University of Glasgow
Email: Paul.shiels@glasgow.ac.uk
The sum total of life course exposures creates an exposome that has a significant impact on age related health. Understanding the interplay between exposome factors and the (epi) genome, offers insights into the ageing process. This has been used as part of a renaissance of a natural sciences approach incorporating biomimetics, to harness evolution through natural selection in other species, to help improve age-related human health span. In particular, this has indicated leverage of the activities of the Nrf 2 gene to enhance health span, via re-introduction of the classical Hippocratic ‘Food as Medicine’ concept, including modulation of the microbiome and the creation of more salutogenic and biophilic environments. I will discuss how this approach integrates with novel and developing senotherapies to tackle age related diseases.

Paul Shiels is Professor of Geroscience at the University of Glasgow. He is a graduate of Trinity College Dublin and received his PhD from Glasgow University. He won an EMBO Long-term Fellowship to work at the Netherlands Cancer Institute.
He is a founder member of GARNER, the Glasgow Ageing Research Network. Paul has established a reputation in the field, developing the kidney as a model of ageing, and is author of over 188 peer reviewed publications and a number of Patents in this sector. Paul has acted as an expert on the Biology of Ageing on national policy advising consortia
including providing evidence to UKXIRA and the All Party Parliamentary Group on Longevity. His research has involved determining exposome factors (socio-economic, psychological, lifestyle and nutrition) and (epi)genomic factors that are required for healthy ageing. He was the first to report on socioeconomic status and nutritional factors affecting epigenetic influences on health. His current research portfolio comprises investigation and application of novel senotherapies, biomimetics and how the microbiome impacts on age related health.
Prof. Shiels has acted as CSO for Pathfinder Cell Therapy PLC and has sat on the Scientific Advisory Boards of TC Biopharm and 4D Pharma. He has a proven track record in public dissemination of his research including the provision of expert commentary for the BBC and ABC TV networks and as a Panellist at the Edinburgh International Science Festival and the Edinburgh International Book Festival.
Nrf2 in stress, aging and age-related diseases
Ioannis Trougakos
Department of Cell Biology and Biophysics, Faculty of Biology, National and Kapodistrian University of Athens, Athens, 15784, GreeceEmail: itrougakos@biol.uoa.gr; http://scholar.uoa.gr/itrougakos/home
Aging is a complex phenomenon caused by the time-dependent loss of cellular homeodynamics and consequently of physiological organismal functions. This process is affected by both genetic and environmental (e.g., diet) factors, as well as by their constant interaction. The balanced functionality of (among others) cellular antioxidant and proteostatic modules is central to genome, proteome and mitochondrial stability. The antioxidant response system comprising (among others) the ubiquitously expressed NFE2-related transcription factor 2 (NRF2) and its redox-sensitive cytoplasmic inhibitor Kelch-like ECH-associated protein 1 (KEAP1) defends tissues against oxidative stress, thereby protecting against pathologies that relate to DNA, protein, and/or lipid oxidative damage. Here we will present our studies aiming to reveal dose-, tissue- and disease-dependent NRF2 function by using as an in vivo experimental platform Drosophila melanogaster. Our research efforts parallel an extensive screening program for the identification of natural products (e.g., extracts or small molecules) from various sources of the biosphere that can be used in a translational intervention as NRF2 activators.
Ioannis Trougakos obtained his Ph.D. in Cellular-Developmental Biology from the National and Kapodistrian University of Athens (NKUA), Greece. He has worked as Research Scientist at EMBL, Germany, CBM “Severo Ochoa”, Spain and at NHRF, Athens, Greece; he was also research visitor at EMBL and at the Netherlands Cancer Institute. Dr. Trougakos was elected Research Lecturer at NHRF and currently he serves as Professor and Director of the “Cell Biology” lab at the Faculty of Biology, NKUA. Dr. Trougakos has published articles (>180) in high-ranking journals, chapters in international books and he co-authored an academic book (citations ~18500; h-Index = 44 / i10-index = 120); he is also co-inventor in several patents. His group is funded by private (GR, EU, USA) and public (GR, EU) entities; also, the group participates in contractual activities with the Industry.
Role of NRF2 signaling pathway on NLRP3 inflammasome regulation
Santiago Cuevas Gonzales, Spain
BioMedical Research Institute of Murcia (IMIB-Arrixaca), Murcia (Spain)
E-mail: Santiago.cuevas@imib.es
Inflammation-mediated inflammasome activation is associated with many diseases, including asthma, cancer, chronic inflammatory diseases, atherosclerosis, diabetes and kidney diseases. Pyroptosis is a lytic type of programmed cell death involved in inflammation, as well as a key fibrotic mechanism that is critical in the development of the pathology of several diseases such as kidney diseases. Pyroptosis is induced by the cleavage of Gasdermins by various caspases and is executed by the insertion of the N-terminal fragment of cleaved Gasdermins into the plasma membrane, creating oligomeric pores and allowing the release of diverse pro-inflammatory products into the extracellular space. Inflammasomes are multiprotein complexes leading to the activation of caspase-1, which will cleave Gasdermin D, releasing several pro-inflammatory cytokines; these results in the initiation and amplification of the inflammatory response, and this mechanism have been associated to several inflammatory diseases.
The efficacy of Gasdermin D cleavage is reduced by a change in the redox balance. Recently, several studies have shown that the attenuation of reactive oxygen species (ROS) production induced by antioxidant pathways results in a reduction of renal pyroptosis. Our preliminary results showed how inflammasome activation is associated with chronic kidney disease (CKD) and the capacity of antioxidants, particularly Nrf2 activators, to inhibit inflammasome activity in bone marrow mouse macrophages.
We show the potential influence of the deregulation of redox balance on inflammasome activity and pyroptosis and point out Nrf2 activators as a novel therapeutic approach for the treatment of autoimmune diseases associated with Inflammasome activation

Santiago Cuevas Gonzales has 22 years of experience in the academy and industry in basic, translational and clinical research studying the pathways involved in the regulation of oxidative stress and inflammation in the pathogenesis of hypertension, cardiovascular and renal diseases. Ten years of research experience in the United States on the field. At the present, I am a Principal Investigator Miguel Servet in the Institute of Biomedical Research in Murcia (IMIB). I am a team leader in the Unit of Molecular Inflammation at the IMIB led by Dr. Pablo Pelegrin, where several groups study the molecular mechanism involved in inflammasome regulation and its role in the pathogenesis of several diseases.
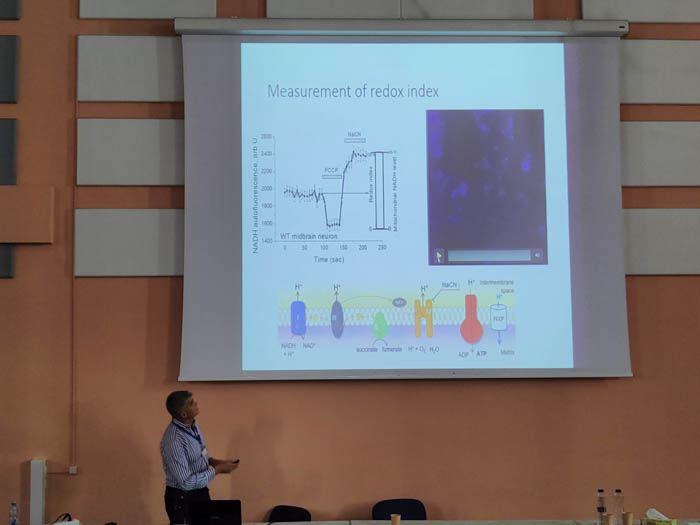
Regulation of energy metabolism in brain by Nrf2: implication to neurodegeneration and epilepsy
Andrey Y. Abramov
Department of Clinical and Movement Neurosciences, UCL Queens Square Institute of Neurology, Queen Square, London, UK
Email: a.abramov@ucl.ac.uk
Energy-producing organelles mitochondria are involved in a number of cellular functions. Deregulation of mitochondrial function due to mutations or effects of mitochondrial toxins is proven to be a trigger for diverse pathologies, including neurodegenerative disorders. Despite the extensive research done in the last decades, the mechanisms by which mitochondrial dysfunction leads to neuronal deregulation and cell death have not yet been fully elucidated. Brain cells are specifically dependent on mitochondria due to their high energy demands to maintain neuronal ion gradients and signal transduction, and also, to mediate neuronal health through the processes of mitochondrial calcium homeostasis, mitophagy, mitochondrial reactive oxygen species production and mitochondrial dynamics. Most of the neurodegenerative disorders and some of neurological conditions such a epilepsy characterised by mitochondrial disfunction which results in energy deprivation and oxidative stress and neuronal loss. Nrf2 controls major endogenous antioxidant pathways and also support mitochondrial metabolism by substrates. We have found that pharmacological activation of Nrf2 restore energy metabolism and increase the level of GSH in the familial forms of Parkinson’s disease (PINK1 and SNCA triplications) and protect neurons against cell death. In epilepsy, KEAP1 inhibition (activation of Nrf2) is neuroprotective and suppresses the development of seizures. However, the most effective treatment of the epilepsy was the combination of the Nrf2 activation (pharmacological Keap1 inhibition) and inhibitor of NADPH oxidase. Importantly, this combination completely restore altered mitochondrial membrane potential, decrease seizure-induced ROS production and prevented the development o f spontaneous seizures. Thus, Nrf2 is one of the most promising target for treatment of the neurodegenerative disorders and epilepsy.

Andrey Y. Abramov is a Professor at the Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology. He studies the role of mitochondria, calcium signalling and redox biology in physiology of the Central nervous system and in the mechanism of the pathology of neurodegenerative disorders. In the last decade in collaboration with Professor Dinkova-Kostova we identified novel and underestimated role of Nrf2 in mitochondrial bioenergetics
NRF2 and iron metabolism in acute stress
Iveta Bernatova
Department of Experimental Hypertension, Institute of Normal and Pathological Physiology, Centre of Experimental Medicine, Slovak Academy of Sciences, Bratislava, Slovak Republic
E-mail: Iveta.Bernatova@savba.sk
Iron is essential in many metabolic processes. In the organism, iron absorption, storage and usage are highly regulated. The main regulator of systemic iron homeostasis is hormone hepcidin primarily secreted by the hepatocytes, and to a lesser extent also in the heart. Intracellular iron metabolism is regulated predominantly through iron-regulatory protein. In addition, iron metabolism was shown to be modulated via NRF2 dependent regulation of several genes, such as ferritin heavy and light chains, transferrin, ferroportin and heme oxygenase-1. Iron metabolism can be altered by chronic stress, an etiological factor in the development of cardiometabolic diseases including heart failure, hypertension or non-alcoholic fatty liver disease. Because there is a crosstalk between the heart and liver, the liver disorders can induce cardiovascular complications and vice versa that has significant clinical impact. This presentation will demonstrate how repeated acute stress induced by air jet alters relative iron content in the heart and liver as well as nitric oxide and superoxide productions in Wistar-Kyoto rats. In addition, the presentation will demonstrate changes in the expressions of the certain genes involved in iron homeostasis and their relation to NRF2.

Iveta Bernatova is a senior scientist, Head of the Department of Experimental Hypertension and a Member of the Management Board of Centre of Experimental Medicine, v.v.i., Slovak Academy of Sciences, Bratislava, Slovakia. She studied Biochemistry (Diploma in 1991), holds Ph.D. from Chemistry (1997) and a title Doctor of Science (D.Sc.) in the field of Ani mal Physiology (2009). Her research is focused on the regulatory mechanisms of blood pressure in various experimental models of hypertension and the ways of prevention and treatment of high blood pressure with special attention paid to the role of nitric oxide and oxidative stress in regulation of blood pressure and of vascular function. Significant part of her research is focused on the role of chronic social stress in development of hypertension and on mechanisms of adaptation to stress. The most recent studies are focused on the research of iron metabolism in the heart and liver, the role of NRF2 in regulation of iron metabolism in rats with normal and high blood pressure as well as on the effect of the acute and chronic stress in NRF2-mediated mechanisms. She is the author of 120 peer reviewed publications in extenso with 2000 citations.
NRF2 and aquaporins in chronic stress
Monika Mlinarić, Lidija Milković, Ana Čipak Gašparović
Institute Ruđer Bošković, Croatia
Email: acipak@irb.hr
Aquaporins, at first described as the cellular plumbing system, are membrane pores that regulate the flux of water and other small polar molecules across the cell membrane. There are 13 human aquaporins, AQP0 to AQP12, each with its own specificity to certain substrates and cellular location. Due to their substrates, aquaporins play a role in cell migration, adhesion, proliferation as well as in water homeostasis. Some aquaporins are specialized in H2O2 transport and are therefore called peroxiporins. Peroxiporins are now recognized as a regulatory factor in oxidative stress response. Even more, the disturbances in the expression of aquaporin are found in different pathologies. This presentation will focus on peroxiporins overexpressed in cancer, their role in chronic stress, and providing a link in antioxidative response with special emphasis on NRF2.

Ana Čipak Gašparović is a Senior Research Associate in the Laboratory for Oxidative Stress, Division of Molecular Medicine at the Ruđer Bošković Institute in Zagreb, Croatia. Her research focuses on the role of oxidative stress and antioxidative response in the resistance to cancer treatment. Recently, her research included aquaporins in breast and colon cancer. Special emphasis is given to peroxiporins, specific aquaporins which, in addition to water and glycerol, channel hydrogen peroxide, and as a consequence contribute to oxidative and antioxidative response of the cell. She is interested in the regulation of NRF2 pathway in response to peroxiporins, and their influence on the development of therapy resistance.
- 16:00 - 16:30 Coffee break.
- 16.30-18:30. Session II. Chair: Sermin Genc
Regulation of glucose uptake and metabolism in brain by Nrf2
Noemi Esteras1*, Thomas S. Blacker2, Michael R. Duchen2, Albena T. Dinkova-Kostova3, Andrey Y. Abramov1
1 Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, University College London, UK.
2 Research Department of Cell & Developmental Biology. University College London, UK.
3Jacqui Wood Cancer, Division of Cellular Medicine, School of Medicine, University of Dundee, Scotland, UK & Departments of Medicine and Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, USA
E-mail: n.gallego@ucl.ac.uk
Nrf2 has been shown to enhance both energy metabolism and antioxidant defence as part of its cytoprotective activity. Both mechanisms are particularly important in the brain, due to its high energy demands and intense oxidative activity, which also rely on a complex interaction between neurons and astrocytes. Energy production and antioxidant defence employ NADH and NADPH respectively as metabolic cofactors, and both are generated in separate pathways of glucose metabolism. We have explored how Nrf2 orchestrates glucose utilization between both pathways under different conditions, employing glio neuronal cultures from wild-type, Nrf2-knockout and Keap1-knockdown mice. To this end, we have used advanced microscopy imaging of single live cells, including multiphoton fluorescence lifetime imaging (FLIM) to discriminate between NADH and NADPH in neurons and astrocytes. In this presentation we will show how Nrf2 activation leads to a higher rate of glucose uptake in both brain cells types, and prioritizes the metabolism of glucose for mitochondrial NADH and energy production, especially in astrocytes, with a much smaller contribution to promote NADPH synthesis for antioxidant pathways. This highlights the role of Nrf2 in modulating glucose metabolism in the brain in different scenarios and the importance of astrocytic Nrf2 to maintain brain redox balance and energy homeostasis.

Noemí Esteras graduated in Pharmacy (2007) and obtained a PhD in Biochemistry and Molecular Biology (2012) from Complutense University of Madrid, Spain; and since 2019 is a Senior Research Fellow at Queen Square Institute of Neurology, University College London, UK. Her work has focused in understanding the molecular mechanisms leading to neurodegeneration, and particularly, the interaction of mitochondria, oxidative stress and calcium signalling in the pathogenesis of disease. She is very interested in the role of Nrf2 as a modulator of mitochondrial function, both in brain physiology and as a therapeutic strategy in neurodegeneration, and has published several original and review articles on the topic.
Neuroprotective glia DJ-1 and function via Nrf2 pathway
Kari Espolin Fladmark
Department of Biological Science, University of Bergen, Bergen, Norway
E-mail: Kari.Fladmark@uib.no
The park7 encoded DJ-1 is a multifunctional protein with particular role in oxidative stress regulation. A non-functioning DJ-1 is associated both to familiar Parkinson´s disease and spontaneous neurodegenerative diseases suggesting that DJ-1 may have a general neuroprotective function. In particular, glial DJ-1 seems to be highly important in order to protect neighbouring neurons. To elucidate the driving mechanisms behind glial DJ-1s neuroprotective role we have established knock-out and transgenic zebrafish models of DJ-1. This presentation will show the response to DJ-1 loss and re-insertion of glial DJ-1. d energy homeostasis.

Kari Espolin Fladmark is a cell biologist and professor at Department of Biological Science at University of Bergen, Norway. She has had research stays at Un iversity of Ghent (2001), University of Pittsburgh (2010/11) and University of Sheffield (2017/18). She established and was the leader of the university core facility in proteomics from 2003-2007. The primary focus of her research group is glial-neuronal crosstalk in which both cell culture and zebrafish are used. A broad range of methods are established including MS-based metabolomics and proteomics, live imaging, and behavior analysis. She has published 53 original papers, four reviews, two book chapters and one patent.
NRF2 and protein quality control systems in Alzheimer-like pathologies
Fabio Di Domenico
Department of Biochemical Sciences, Sapienza University of Rome, Rome, Italy
E-mail: Fabio.didomenico@uniroma1.it
Increased oxidative stress (OS), due to mitochondrial dysfunction and the failure of antioxidant responses, represents an early signature of Alzheimer Disease (AD) neuropathology, triggering protein oxidative modification and the build-up of toxic aggregates. Protein quality control (PQC) systems are involved in the surveillance of protein folding/degradation and reduce the accumulation of neurotoxic damaged proteins. Data from our laboratory demonstrated in Down syndrome (DS) and AD brain the impairment of PQC systems that may promote the escape of unfolded/misfolded proteins from protein homeostasis mechanisms favoring aberrant protein aggregation. Beyond the alteration of the ubiquitin proteasome system (UPS) and of autophagy we recently reported the aberrant induction of the unfolded protein response (UPR) during the neurodegenerative process. We demonstrated that the increased oxidation of BiP may lead to the selective detrimental over induction of the PERK branch of the UPR in AD-like neuropathology. The dysregulation of PERK/eIF2a axis was associated with the reduction of translation and with the increased expression of the pro apoptotic signals. Surprisingly, we also observed the uncoupling between PERK and Nrf2 response, due to early Bach1 overexpression in DS and to aging in AD. Data collected in AD and DS human brain were corroborated by the analysis of murine models and of blood derived primary cells and cultures. The subsequent pharmacological manipulation of PERK in DS models was able to rescue proteostasis and to reduce the build-up of oxidative damage as effect of Nrf2 signalling rescue. Our results suggest that the failure to regulate the PERK pathway and its uncoupling with Nrf2 is both cause and effect of increased OS and may represent an essential step in promoting aberrant proteostasis in AD-like pathologies.

Fabio Di Domenico is Full professor of Biochemistry at Sapienza University of Rome. He obtained his PhD degree in Biochemistry in 2009. Before gaining his current position, he performed h is research under the supervision of Prof. Butterfield at University of Kentucky, where he have been involved in the application of redox proteomics studies. His research is currently focused in understanding the mechanisms that associates increased oxidative stress and defective proteostasis in the development of Alzheimer-like dementia. Collected data from his laboratory postulate that aberrant proteostasis, observed in both Alzheimer and Down syndrome patients, is strictly associated with the increase of oxidative damage as result of compromised antioxidant response and faulty protein degradative systems. Recently, his studies revealed that the chronic induction of the unfolded protein response and its aberrant relationship with Nrf2 response hold a prominent role in the development of dementia in the brain from Alzheimer and Down Syndrome patients.
NRF2 in the context of inflammation in Alzheimer’s disease
Gina Manda1*, Maria Dobre1, Elena Milanesi1, Catalina Anca Cucos1, Elena Mihaela Dragnea1, Gerard Piñol-Ripoll2, Antonio Cuadrado3
1 Radiobiology Laboratory, “Victor Babes” National Institute of Pathology, Bucharest, Romania
2Unitat Trastons Cognitius, Hospital Universitari Santa Maria-IRB Leida, Lleida, Spain
3 Department of Biochemistry, Faculty of Medicine, Autonomous University of Madrid, Madrid, 28049, Spain
E-mail: gina.manda@gmail.com; gina.manda@ivb.ro
It is known that chronic low-grade inflammation and oxidative stress are underlying many chronic diseases, including neurodegeneration. Important gene expression changes related to NFkB mediated inflammation and redox disturbances, including the activation of some of the NRF2 target genes, have been previously highlighted by us in the blood of 38 patients with mild Alzheimer’s disease (AD), as compared with 38 age- matched controls presenting similar comorbidities [1]. In the search of a reliable animal model of AD for drug development (NRF2- and inflammation targeted therapies) we investigated the expression profile of 168 inflammation and redox genes in the blood and hippocampus of double transgenic. We identified distinctive gene expression profiles, inflammation genes with significantly modified expression being detected in blood, while redox genes disturbances dominating in the hippocampus of transgenic AD mice. Interesting, the GSR gene, which is target of the transcription NRF2, was found significantly over-expressed both in the blood of mild AD patients and AD mice, and was associated with an increased oxidative activity in mice, as seen from the over-expression of the redox-responsive OSGIN1 gene. The u-regulation of these redox genes was detected early during disease progression, but showed no correlations with age. Additionally, we have found that the NRF2 target gene TXN1 was over-expressed and correlated well with age and disease progression in the hippocampus of AD mice.
Concluding, based on gene expression profiles, we have placed NRF2 target genes in a network of redox and inflammation genes in APPV717I × TAUP301L mice, that partially overlaps gene expression changes in the blood of AD patients, at least at the level of the GSR gene. The identified panels of genes with a common trend of expression changes in AD mice and patients is useful for overcoming the generally low animal-to human translational success

Gina Manda is the head of the Radiobiology laboratory at “Victor Babes” National Institute of Pathology, Bucharest, Romania. She is currently studying the molecular mechanisms underlying: i) low-grade inflammation and disturbed redox signaling in chronic diseases (Alzheimer’s disease), and ii) the responses of normal and tumor cells elicited by radiation exposure in experimental settings relevant for anti cancer therapies (radio- or photodynamic therapy) or for space medicine. One of the main interests is to develop at preclinical level therapies that target NRF2, in order to: i) increase the efficacy of anti-cancer therapies using targeted NRF2 inhibitors; ii) counteract using NRF2 activators the deleterious effects of galactic cosmic radiation on astronauts during long-term travel in the deep space, an issue that is highly relevant in this era of intensive preparations for space exploration.
Differential expression of NRF2 signaling in neurons and astrocytes associated with α– synuclein: implications for Parkinson’s disease
Isabel Lastres-Becker
Instituto de Investigaciones Biomédicas “Alberto Sols” UAM-CSIC, Arturo Duperier, 4, Department of Biochemistry, School of Medicine, Institute Teófilo Hernando for Drug Discovery, Universidad Autónoma de Madrid , 28029 Madrid, Spain
E-mail: ilbecker@iib.uam.es
Parkinson’s disease is the second most prevalent neurodegenerative disease and the first associated with motor impairment. It is a disease in which neurodegeneration of dopaminergic neurons in the Substantia Nigra pars compacta occurs, accompanied by inflammation and oxidative stress. The transcription factor NRF2, among the multiple functions in which it is involved, is the main modulator of redox homeostasis and regulates inflammatory processes. We have previously described how a lack of NRF2 enhances neurodegeneration, neuroinflammation, and oxidative stress in murine models of PD. Furthermore, treatment with an NRF2 inducer, dimethyl fumarate, was able to slow down the degenerative process in this disease. Our studies also indicated that NRF2 activation occurred preferentially in astrocytes and microglia. We, therefore, studied NRF2 expression in neurons and astrocytes in the context of PD in more detail. Our results suggest a differential expression between neurons and astrocytes of the NRF2 signaling pathway in the context of synucleinopathy in PD. These results may have a very relevant impact on the possible use of NRF2 activators as therapeutic strategies for PD.

Isabel Lastres-Becker has more than 20 years of experience in the field of neurodegenerative diseases. Her work is focused on the molecular bases of neurodegeneration related to proteinopathy, neuroinflammation, and oxidative stress. She is the head of the laboratory of “New therapeutic strategies in neurodegenerative diseases: Parkinson’s disease (PD), tauopathies and amyotrophic lateral sclerosis (ALS)”. Since 2008 she has been interested in the implication of the transcription factor NRF2 in neurodegeneration, endorsed by 20 publications in relevant international journals. Her wide research background covers the most prevalent neurodegenerative diseases, looking for reliable markers of progression that can also serve as drug targets for modulating neurodegeneration such as NRF2. She is engaged in developing advanced new drugs and appropriate technology to establish treatments for those diseases, based on NRF2.
Redox, mitochondrial and NRF2 imbalance are present in C9ORF72-related amyotrophic lateral sclerosis
José Jiménez-Villegas, Janine Kirby, Ana Mata, Susana Cadenas, Martin R Turner, Andrea Malaspina, Pamela J Shaw, Antonio Cuadrado and Ana I Rojo*
*Department of Biochemistry, Medical College, Autonomous University of Madrid (UAM), Madrid, Spain.
Parkinson’s disease is the second most prevalent neurodegenerative disease and the first associated with motor impairment. It is a disease in which neurodegeneration of dopaminergic neurons in the Substantia Nigra pars compacta occurs, accompanied by inflammation and oxidative stress. The transcription factor NRF2, among the multiple functions in which it is involved, is the main modulator of redox homeostasis and regulates inflammatory processes. We have previously described how a lack of NRF2 enhances neurodegeneration, neuroinflammation, and oxidative stress in murine models of PD. Furthermore, treatment with an NRF2 inducer, dimethyl fumarate, was able to slow down the degenerative process in this disease. Our studies also indicated that NRF2 activation occurred preferentially in astrocytes and microglia. We, therefore, studied NRF2 expression in neurons and astrocytes in the context of PD in more detail. Our results suggest a differential expression between neurons and astrocytes of the NRF2 signaling pathway in the context of synucleinopathy in PD. These results may have a very relevant impact on the possible use of NRF2 activators as therapeutic strategies for PD.

Ana I Rojo studied Biochemistry at the Autonomous University of Madrid (Diploma in 2001), holds a PhD in Biochemistry (graduated in 2006), and since 2017 is professor in Biochemistry at the Autonomous University of Madrid (Faculty of Medicine). As professor, she has participated in multiple teaching activities for the degrees of Biochemistry, Medicine, and Nursing, with special focus on research training. She has been holder of different competitive fellowships and contracts. Her professional career is focused on the study of the molecular basis of neurodegenerative diseases and in the search for novel brain protective therapies. Nowadays, she is exploring the role of NRF2 in the pathogenesis of Alzheimer’s disease and lateral amyotrophic sclerosis. She has published over 49 primary and review articles and participated in more than 30 congress. She has been a member of the organizing committee of four scientific meetings.
- 16.30-18:30. WG meetings Chair: WG leaders
October 14th
- 9:00 - 11:00. Session III. Chair: Harry Van Goor
NRF2 and multiple sclerosis
Đorđe Miljković
Department of Immunology, IBISS, University of Belgrade, Serbia
E-mail: djordjem@ibiss.bg.ac.rs
Immune cells activated at the periphery infiltrate the central nervous system (CNS) and cause inflammation within the CNS in multiple sclerosis. As the consequence, demyelination and neurodegeneration occur, leading to various neurological deficits in the patients. The interest in NRF2 research related to pathogenesis of multiple sclerosis, stems from the central role that this transcription factor has in redox regulation of immune and CNS cells. This role is, therefore, extremely important for regulation of inflammatory properties of immune cells and for neuroprotection. Knowing the exact role of NRF2 in particular cells during the pathogenesis of multiple sclerosis, as well as of molecular mechanisms behind modulation of NRF2 activity is crucial for design of novel therapeutics that would contribute to the treatment of this disease. Here, current state of knowledge on NRF2 in multiple sclerosis will be presented, along with perspectives for future research will be outlined.

Đorđe Miljković is research professor and head of the Department of
Immunology at the Institute for Biological Research “Siniša Stanković”,
University of Belgrade. He studies cellular and molecular mechanisms
involved in pathogenesis of autoimmune diseases. His current main
research inte rests are: role of gut immune cells in autoimmunity,
mechanisms of autoimmunity progression/regulation, cell-based therapy
of autoimmunity, modulation of autoimmune diseases by synthetic and
natural compounds.
Trisomy21 and aberrant Bach1/Nrf2 axis: implications for neurodegeneration
Marzia Perluigi
Department of Biochemical Sciences, Faculty of Pharmacy and Medicine, Sapienza University of Rome, Rome (Italy)
E-mail: Marzia.perluigi@uniroma1.it
Several studies support the implication of oxidative stress (OS) in phenotypical alterations of Down Syndrome (DS) subjects. The mapping of HSA21 showed the involvement of several genes, such as SOD-1, BACH1, APP, CBR and S100B, in the over-production of ROS in DS individuals and animal models. We investigated the role of BACH1 in the brain and its implication in the failure of antioxidant response, both in Ts2cje mice, as a mouse model of DS, and human DS lymphoblastoid cell lines (LCLs). Our results revealed that BACH1 overexpression alters the BACH1/NRF2 ratio in the nucleus and disturbs the induction of antioxidant response genes ultimately resulting in the accumulation of oxidative damage both in Ts2Cje mice and LCLs. Based on this evidence, we tested Caffeic Acid Phenethyl Ester (CAPE) and the synthetic analogue VP961, which have been proven to modulate NRF2 activity. We showed that CAPE and VP961 administration to DS LCLs was able to promote NRF2 nuclear translocation, which resulted in the amelioration of antioxidant response. Overall, our study supports the hypothesis that BACH1 triplication in DS subjects is implicated in the alteration of redox homeostasis that might contribute to accelerated neurodegeneration, ultimately resulting in early onset Alzheimer disease.

Marzia Perluigi is Professor of Biochemistry at the Department of
Biochemical Sciences, Medical School Sapienza University of Rome”. The
major research interest is the study of the role of oxidative stress in
Down Syndrome (DS) and Alzheimer Disease (AD). Projects involve both
the analysis of post-mortem brains, biological fluids and cellular and
animal models of the diseases. In particular, current projects focus on
the identification of trisomic genes that are involved in the “oxidative
stress phenotype” of DS individuals. Among these triplication of BACH1
may offer the opportunity to understand the role of Nrf2 in the
neurodegenerative process ultimately leading to early onset AD.
The interplay of NRF2/KEAP1 axis and miR-34a in endothelial cell function and aortic pathology
Anna Grochot-Przeczek
Department of Medical Biotechnology, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, Krakow, Poland
E-mail: anna.grochot-przeczek@uj.edu.pl
An abdominal aortic aneurysm (AAA) is a life-threatening, age-associated dilatation of the abdominal aorta. Endothelial cell (EC) dysfunction is proposed to play a role in AAA formation. NRF2 and miR 34a are crucial regulators of ageing and EC biology. Thus, we hypothesized that they might have a decisive influence on the physiology of blood vessels and AAA development. NRF2 transcriptional deficiency in mice (NRF2 tKO) caused ultrastructural changes in the aorta and led to its premature ageing. Formation of AAA was favored in NRF2 tKO animals compared to wild-type counterparts
(NRF2 WT). The level of miR-34a increased in the EC layer of NRF2 tKO aorta, and in response to treatment with angiotensin II (Ang II), a hypertensive peptide, in serum and ECs. We efficiently rescued EC NRF2-dependent premature ageing and AAA formation in NRF2 tKO mice using the EC specific knockout of miRNA-34a, which implies the significance of NRF2, miR-34a and intimal layer in the susceptibility to AAA. However, contrary to previously postulated mechanisms, in our hands the maintenance of specialized functions of ECs was not the primary determinant of aneurysm formation. We propose instead that EC proliferation protects against AAA and can confer aneurysm stability.

Anna Grochot-Przeczek works at the Department of Medical
Biotechnology, Faculty of Biochemistry, Biophysics and Biotechnology, at
Jagiellonian University in Krakow, Poland. She studies the molecular
mechanisms regulating the function of endothelial cells and blood
vessels with a focus on ageing and S-nitrosation. Currently, she tries to
recognize the significance of NRF2/KEAP1 imbalance and loss of
proteostasis in the function of blood vessels.
Pharmacologic and genetic activation of Nrf2 confers anti-fibrotic effects in liver fibrosis
Sharadha Dayalan Naidu
Cellular and Systems Medicine, Ninewells Hospital and Medical School, University of Dundee, Scotland, United Kingdom
E-mail: szdayalannaidu@dundee.ac.uk
Chronic liver injury and inflammation, often resulting in oxidative stress burden, cause liver fibrosis, characterized by excessive scarring. Unresolved hepatic fibrosis can lead to cirrhosis, and ultimately to liver failure. To date, there are no clinically approved drugs to treat liver fibrosis. Oxidative stress triggers Tgf-β1-mediated transdifferentiation of quiescent hepatic stellate cells (HSCs) into proliferative myofibroblasts, the main cells responsible for extracellular matrix deposition and scar formation. One mechanism by which cells combat oxidative stress is through induction of transcription factor nuclear factor-erythroid 2 p45-related factor 2 (Nrf2). We hypothesized that Nrf2 activation in hepatic stellate cells (HSCs) can counteract oxidative and inflammatory stress, thereby inhibiting fibrogenesis. Pre-treatment or co-treatment with two types of Nrf2 activators, a cyanoenone triterpenoid (TP) and a protein-protein interaction inhibitor (PPI) reduced the Tgf-β1- induced expression of pro-fibrotic markers. In Tgf-β1 transdifferentiated HSCs, only TP, but not PPI, was effective. The anti-fibrotic activity of the PPI was Nrf2-dependent, whereas the anti-fibrotic activity of the TP was partially Nrf2-dependent and superior to that of the PPI. Considering that TP are in clinical trials and have well-defined safety profiles in humans, our findings suggest that these compounds hold promise as potential therapeutic agents for liver fibrosis.

Sharadha Dayalan Naidu graduated with a PhD in Medicine at the
University of Dundee in 2016. She joined the Cullman Chemoprotection
Centre as a postdoctoral research scholar at the Department of
Pharmacology and Molecular Sciences at Johns Hopkins Univers ity in
2017, where her work was focused on identifying the mechanism(s) of
action of novel compounds. She returned to Scotland in 2019 and, has
since continued her post-doctoral research in Professor Albena T.
Dinkova-Kostova’s laboratory. Currently, her work is focused on looking
at the benefits of using various classes of Nrf2 activators to treat liver
fibrosis. She has published over 25 original and review articles, which are
primarily focused on Nrf2-Keap1 biology.
Targeting the NRF2/β-TrCP axis in liver disease
Antonio Cuadrado
Department of Biochemistry, Medical College, Autonomous University of Madrid, Madrid, Spain.
E-mail: antonio.cuadrado@uam.es
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of liver disease in developed countries. Is conversion into non-alcoholic steatohepatitis (NASH) involves progressive liver degeneration that presents with chronic low grade inflammatory, oxidative and metabolic stress. At this time, no medicines specifically for NAFLD or NASH have received approval. Transcription factor NRF2 is a master regulator of homeostatic responses and is emerging as a promising target to prevent or stop NASH progression. We will present a new pharmacological strategy to activate NRF2 selectively in liver, based on the disruption of its interaction with the E3 ligase adapter beta-TrCP. A protein-protein interaction inhibitor of this interaction up-regulates the NRF2 transcriptional signature and down-regulates the NF-kB pathway to promote its anti inflammatory effects. Extensive data will be presented in vitro and in cell culture demonstrating the selectivity of this compound towards the disruption of the NRF2/ -TrCP interaction and attenuation of LPS-induced inflammation in cultured macrophages and liver. We further analyzed the effect of this compound in the STAM model of progressive liver damage by NMR, measuring the fat/water ratio, and histochemistry of oil red (fat) and Sirius red (fibrosis), and correlated inflammatory and metabolic parameters with NRF2 activation. We found that this PPI inhibitor prevented NASH onset. Importantly, mice which were allowed to develop NASH and were then submitted to chronic treatment with this compound for 4 weeks exhibited a significant protection against the development of fibrosis. Our results report an innovative mechanism to activate NRF2 and protect the liver from NASH and fibrosis.

Antonio Cuadrado, is full professor of Biochemistry and Molecular Biology at the Department of Biochemistry, Medical School, Autonomous University of Madrid. He obtained his PhD degree in Biology in 1985 and enjoyed several postdoctoral stays in the National Cancer Institute-NIH with the help of Fulbright and Fogarty fellowships.
He established his independent laboratory as Professor of Biochemistry in 1997 with a main interest on the study of molecular mechanisms involved in initiation and progression of chronic diseases. For the past years his main lane of research has been the validation of transcription factor NRF2, master regulator of cellular homeostasis as a new therapeutic target in chronic diseases with particular emphasis in neurodegenerative diseases (Alzheimer and Parkinson) and in fatty liver diseases. His current interest is the development of new NRF2-modulating drugs. Dr. Cuadrado has published over 160 primary and review articles, of which more than 80 are related to the role of NRF2 in physiological and pathological responses to disease.
Gene-environment interaction in NRF2 signaling: the case of thyroid and iodine
Dionysios V. Chartoumpekis, Panos G. Ziros and Gerasimos P. Sykiotis
Service of Endocrinology, Diabetology and Metabolism, Lausanne University Hospital (CHUV), Switzerland
E-mail: dionysios.chartoumpekis@chuv.ch
The thyroid is an organ that relies heavily on the presence of reactive oxygen species (ROS) for its physiological function, namely the synthesis of thyroid hormones that involves oxidation of iodine. Either lack or excess of iodine intake can lead to aberrant thyroid function and increased thyroid size (goiter) in experimental models as well as in humans. We have shown that Nrf2 orchestrates the antioxidant response in thyroid upon iodine overload and regulates the expression, iodination and
processing of thyroglobulin, the major thyroid protein and precursor of thyroid hormones. We have also shown that constitutive activation of Nrf2 pathway by Keap1 knockdown (KD) led to diffuse goiter in mice. To further investigate the thyroid transcriptomics signature of gain-of-Nrf2 function in the presence of basal or excess iodine concentrations, an RNA-seq analysis was performed. As expected, cytoprotective/antioxidant pathways were inducted upon exposure to iodine, and this was more pronounced in Keap1KD mice. In addition, cell cycle, cell proliferation and DNA replication pathways were slightly upregulated upon exposure to iodine in WT mice. In Keap1KD mice, the baseline expression of the respective genes was higher than in WT mice, and their induction by iodine was also much stronger. The enrichment of these pathways in Keap1KD mice could at least partially explain their goiter phenotype. Given that there are reported clinical cases with hereditary goiter in families with germline KEAP1 loss-of-function mutations, our findings establish the mouse as a suitable model to fully elucidate the potential cross-talk between cell proliferation pathways and Keap1/Nrf2 signaling after exposure to various concentrations of iodine.

Dionysios Chartoumpekis MD, PhD is an endocrinologist and researcher in Lausanne University Hospital. After defending his PhD thesis at the University of Patras, Greece on the role of Nrf2 as a mediator of the antioxidant effects of statins, he did postdoctoral research in the Kensler Lab at the University of Pittsburgh, USA on the crosstalk of Nrf2 with metabolic processes in obesity and type 2 diabetes. He is interested in studying the roles of Nrf2 at the intersections between the antioxidant response and other cellular pathways in endocrine tissues. Based on a better understanding of how these interactions affect the respective phenotypes, he hopes to target them to prevent or treat metabolic and endocrine diseases.
- 11:00 - 11:30 Coffee break.
- 11:30 - 13:30. Session IV. Chair: Joana Miranda
Skin barrier composition in epidermis of aged mice deficient in NRF2
Michaela Sochorova, Ionela-Mariana Nagelreiter, Christopher Kremslehner, Francesca Ferrara, Katerina Vavrova, Florian Gruber*
*Department of Dermatology, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Wien;
Email: florian.gruber@meduniwien.ac.at
Nrf2 is a major regulator of the antioxidant response, and it’s activation downstream signaling have been reported to be impaired in aging in several tissues. In the epidermis of the skin, Nrf2 has additional relevance, as disturbance in it’s function, especially superphysiological activation of this transcription factor result in severe impairment of the epidermal barrier function and inflammation. We here investigated whether genetic deficiency in Nrf2 would affect the composition and function of the epidermal barrier in chronological aging of mice. To this end we performed lipidomic, immunohistochemical and functional studies on murine tail skin of young (6 +/-1 months) and aged (18 +/-2 months) mice. The thinning of the epidermis that could be observed in the aged wildtype mice was not observed in the aged Nrf2 deficient mice. This unexpected finding went in hand with an increased rate of proliferation of basal epidermal keratinocytes. We investigated epidermal gene expression and found that, as expected Nrf2 target genes including Nqo1 showed reduced baseline expression in knock out epidermis of both age groups. Expression of the epidermal LaminB1 was strongly reduced in aged wildtype mice, and increased from a low level in aged Nrf2 deficient mice, which showed also increased MMP10 expression. When investigating the three main barrier lipid classes free fatty acids (FFA), cholesterol (Chol) and ceramides (Cer), we found lower overall content in the epidermis of young knockouts as compared to their wildtype counterparts. GC-MS identified that in old animals of both genotypes the total FFA were increased, and this was amplified in the knockouts as was the age related increase in the percentage of unsaturated FFA, most prominently linoleic acid. Outside in barrier function was not measurably impaired. Lack of Nrf2 in mice thus leads to a state of higher proliferation or replenishment of transient amplifying keratinocytes that goes in hand with increased remodeling and FFA synthesis, which may indicate that lack of Nrf2 requires a higher epidermal KC turnover with increasing age.

Assoc. Prof. Priv.-Doz. Mag. Dr. Florian studied Biology and Genetics at the Unviersity of Vienna, and gained, after a spring term at Harvard College, the Master and later the Ph.D. at the Unviersity of Vienna. Moving between the fields of Vascular Biology at the M edical University of Vienna (MUW) and the Cardiovascular Research Center of the University of Virginia, and Dermatology at MUW, he became Associate Professor and gainded his venia docendi in the field of Experimental Dermatology at the MUW.
Main areas of research The interaction of the skin with the environment is a major research topic of the Gruber workgroup. We investigate how the skin and its various cell types cope with intrinsic and extrinsic stressors and with aging. A special focus of our research is on the role that lipids and their oxidation play in skin biology. We investigate how the cytoprotective mechanisms autophagy and the antioxidant response influence the resistance to extrinsic stressors and how they modulate the cellular senescence process. Florian Gruber heads the Christian Doppler Laboratory for Skin Multimodal Imaging of Aging and Senescence, a collaborative research project on skin senescence and exposure to pollution together with Markus Schosserer’s Lab at the University of Natural Resources and Life Sciences of Vienna, Martina Marchetti-Deschmanns Lab at the Technical University Vienna and Chanel (France).
A repurposing strategy for Age-related Macular Degeneration targeting NRF2
Sandra Tenreiro*, Ana Sofia Falcão, Mafalda Lopes da Silva, Pedro Antas, Inês Paz Santos, Miguel C. Seabra
*iNOVA4Health, NOVA Medical School | Universidade NOVA de Lisboa, Lisbon, Portugal
E-mail: stenreiro@nms.unl.pt
The prevalence of all forms of AMD in the USA is similar to that of all invasive cancers combined, and over double of that of Alzheimer’s disease. Currently, it is a serious medical unmet need as the anti- angiogenic antibodies applied intravitreally are a therapeutic alternative only for the late AMD wet form affecting 10% of all patients. However, there is no treatment available for the more common early, intermediate and late “dry” forms of AMD which affect 90% of patients.
The primary cause of pathology in AMD appears to be retinal pigment epithelium (RPE) thinning, accumulation of autofluorescence (lipofuscin) and depigmentation, leading to atrophy. We have developed a model system that recapitulates several AMD features in vitro. Namely, feeding RPE monolayers in culture with photoreceptor outer segments (POS) leads to accumulation of autofluorescent granules (AFGs) similar to lipofuscin in vivo. Notably, our data suggests that AFGs derive from incompletely digested POS-containing phagosomes as they are surrounded by a single membrane containing lysosome markers.
We have developed a model system that recapitulates some AMD features in vitro; feeding RPE monolayers in culture with POS leads to accumulation of autofluorescent granules (AFgs) similar to lipofuscin in vivo, resembling RPE stress and disease. Notably, over-expression of the transcriptional regulators TFEB and NFE2L2/NRF2 can prevent the formation and/or resolve autofluorescent granules. Curcumin analogues C1 and C2, described as inducers of both TFEB and NRF2 or TFEB alone, respectively, were able to significantly decrease uvPOS-dependent AF. Treatment with the NRF2 activator Dimethylfumarate also leads to a significantly decrease in POS-dependent AF, reinforcing NRF2 is a promising therapeutic target for AMD. We are exploring pharmacological NRF2 activators in our RPE models, focusing on repurposing clinically tested drugs. In parallel we are also testing other new NRF2 activators. Moreover, we are dissecting the molecular mechanisms underlying the prevention and/or clearance of autofluorescent granule load in RPE models by macroautophagy and the anti-oxidative stress response.

Sandra Tenreiro research is focused in clarifying the molecular mechanisms of retinal degeneration in the context of ageing diseases, using cellular models retinal pigmented epithelium and 3D retinal organoids, differentiated from human iPSc. ST is a Group Leader at NOVA Medical School, Universidade Nova de Lisboa, Portugal. She holds a PhD degree in Biotechnology since 2001. She was Principal Investigator (PI) and Co-PI in several research projects and has participated extensively in advanced training, supervising post-docs (6), PhD (4) and MSc students (14). Collaborates in several PhD and MSc programs from Universidade Nova de Lisboa but also from Coimbra University. Globally, her scientific career is reflected in 53 peer-reviewed publications with an
H-INDEX of 29 (according to Scopus).
Sorafenib downregulates nuclear factor E2-related factor 2 (Nrf2)-regulated thioredoxin 1 (Trx1) expression impacting cell survival in liver cancer cells
Jordi Muntané
Department of Medical Physiology and Biophysics, University of Seville, Seville, Spain
E-mail: jmuntane-ibis@.es
Hepatocellular carcinoma (HCC) represents 80% of the primary hepatic neoplasms. It is the sixth most frequent neoplasm, the fourth cause of cancer-related death, and 7% of registered malignancies. Although immunotherapy and antiangiogenic combined treatment is emerging as first line therapy, Sorafenib is a useful treatment in the actual pipeline for patients in advanced stage of HCC. The administration of Sorafenib, and other tyrosine kinase inhibitors, is widely associated with mitochondrial dysfunction and the generation of reactive oxygen (ROS) and nitrogen (RNS) species. However, Sorafenib exerts a role as a free radical scavenger assessed by electron paramagnetic resonance, EPR. We also observed that Sorafenib downregulates nuclear factor E2-related factor 2 (Nrf2)-regulated thioredoxin 1 (Trx1) expression in liver cancer cells. In order to elucidate the function of Trx1 in our system, siRNA strategies and/or its overexpression showed that Trx1 induced activation of nitric oxide synthase (NOS) type 3 (NOS3) and S-nitrosation (SNO) of CD95 receptor leading to an increase of caspase-8 activity and cell proliferation, as well as reduction of caspase-3 activity in liver cancer cells. Sorafenib also transiently increased mRNA expression and activity of S- nitrosoglutathione reductase (GSNOR) in HepG2 cells. Different experimental models of hepatocarcinogenesis based on the subcutaneous implantation of HepG2 cells in nude mice, as well as the induction of HCC by diethylnitrosamine (DEN) confirmed the relevance of Trx1 downregulation during the proapoptotic and antiproliferative properties induced by Sorafenib. In conclusion, the induction of apoptosis and antiproliferative properties by Sorafenib were related to Nrf2-regulated Trx1 downregulation that appeared to play a relevant role on SNO of NOS3 and CD95 in HepG2 cells.

Jordi Muntané is Associate Professor in the Department of Medical Physiology and Biophysics, School of Medicine, University of Seville (Spain). The group is deciphering the molecular mechanism related to the antitumoral properties of tyrosine kinase inhibitors (TKIs) in liver cancer cells. In particular, the impact in mitochondrial dysfunction, oxidative and nitrosative stress and cell metabolism, endoplasmic reticulum stress, autophagy and apoptosis in the effectiveness of TKIs in liver cancer cells. The translational impact of the research involves the identification of circulating tumor cells (CTCs), extracellular vesicles (EVs) and miRNA and lncRNA signatures in blood from patients used as prognostic value of the disease and treatment response in patients with advanced HCC. In particular for the participation of our group in
BenBedPhar network we will be focus on: 1) Molecular mechanism of Nrf2 regulation by TKIs. 2) Impact of NRf2-regulated genes in the antitumoral properties of TKIs and 3) Impact of drugs regulating Nrf2 in liver cancer cells.
Identification of novel Cycloastragenol derivatives as potent NRF2 activators that delay replicative senescence
Petek Ballar Kırmızıbayrak1, Erdal Bedir2, Sinem Yılmaz3
1*Department of Biochemistry, Faculty of Pharmacy, Ege University, Bornova, Izmir, Turkey 2 Department of Bioengineering, Izmir Institute of Technology, Urla, Izmir, Turkey
3 Department of Bioengineering, University of Alanya Aladdin Keykubat, Antalya, Turkey
E-mail: petek.ballar@ege.edu.tr
Aging is characterized by the reduced cellular functionality. Several hallmarks contribute to aging, such as loss of proteastasis, telomere attrition, and increased ROS (reactive oxygen species) levels. As redox balance, telomerase, and proteasome are associated with aging and age-related disease, targeting these systems with small compounds has been considered a promising therapeutic approach. Cycloastragenol (CA) is a small molecule telomerase activator derived from the extract of Astragalus membranaceous, a plant widely used in traditional Chinese medicine for the treatment of several diseases such as diabetes, atherosclerosis, and cancer. We recently reported that CA functions at the intersection of NRF2, telomerase and proteasome systems. Furthermore, CA- mediated induction of proteasome and telomerase activity was found to be regulated by NRF2. As at present, poor water solubility, faster metabolic conversion and lower oral bioavailability still restrict the clinical application of CA, it is important to develop new derivatives of CA. This presentation will focus on the effect of novel CA derivates obtained via endophytic fungi-mediated biotransformation. Among the six tested CA derivatives identified as telomerase activators, treatment with some CA derivatives promoted NRF2 and proteasome activation at much lower concentrations than CA in human neonatal epithelial keratinocyte (HEKn) cells. Furthermore, CA and its derivatives not only delayed replicative senescence in young, moderate, and terminated HEKn cells, but also reduced the p16 levels, which increased with senescence. Because of their activity at concentrations as low as 0.1 nM and their function at the intersection of NRF2, proteasome and telomerase systems, selected derivatives would be further studied for their ADMEs (the absorption, distribution, metabolism and excretion).

Petek Ballar Kırmızıbayrak studied Pharmacy at Ege University (2001) and holds a Ph.D. degree in Molecular and Cell Biology from the University of Maryland, Baltimore (2007). She is a full professor in the Biochemistry Department of the Faculty of Pharmacy at Ege University. Her research is mainly focused on Endoplasmic reticulum (ER)- associated protein degradation, Ubiquitin Proteasome System, ER stress, and Unfolded Protein Response. She participated in several national and international research projects as mainly PI or Co-PI. She has published 52 peer-reviewed publications, and worked as an active MC member of several COST actions including CA20113, BM1307, and CA15138.
Drugs repurposing to target Nrf2 function in the Th17/Treg axis
Michel Edwar Mickael, Norwin Kubick, Maruisz Sacharczuk
Institute of Animal Biotechnology and Genetics, Polish Academy of Science, Poland.
Drug repurposing constitutes a lifeline for the drug industry. Production costs of a novel drug can reach 1 billion dollars. The time needed for a single drug to be available for patients is ten years. AI drug repurposing bypasses these hurdles by significantly reducing the costs associated with drug development. As a case study, this presentation covers the progress made to repurpose drugs that can regulate Nrf2 function in the Th17/Treg axis during brain inflammation. This axis constitutes a primary target for drug intervention. Th17 and Tregs belong to the same group of CD4+ T cells. They also share a large section of their transcriptome but their functions are fundamentally different. Th17 cells are known to increase brain inflammation through the production of IL17A, IL17F, IL1, and IL6. Treg cells have an anti-inflammatory effect and are known to inhibit Th17 through various direct and indirect mechanisms. We have found that during various brain conditions, gut Th17 cells infiltrate the brain causing demyelination, astrogliosis, and lesions-like structures in the hypothalamus. Interestingly, we found that Tregs infiltration of the brain is minimal. Using AI we are repurposing several drugs that specifically target Nrf2 pathway in Th17 cells in order to re- differentiate them toward an anti-inflammatory type. To achieve this we are using our novel AI software known as Adera2.0. Previously, using Adera1.0, we showed that zileuton is capable of activating Nrf2 in macrophages subjected to LPS. We have updated our software to more effectively search PubMed for drugs that could be repurposed to target a specific gene. Adera2.0 is composed of three different AI networks. (i) The first network converts every sentence in each PDF into a matrix of 512 values. (ii) The second network measures the relevance between the pathway investigated and every sentence in every PDF searched. (iii) The third network extracts the compound names from each relevant sentence. Using our flow, we have repurposed five new drugs that are currently being experimentally evaluated. Overall our workflow could significantly reduce the time and cost needed for drug production.

Michel Edwar Mickael is an assistant professor at IGHZ, Warsaw, Poland since 2020. Before that, he conducted postdoctoral training in immunology at UAB, Alabama, and Victor Babes institute. His primary interest is using computational and experimental techniques to regulate Nrf2 immunological function.
Carbon nanoparticles for targeted drug delivery
Silvia Giordani
1School of Chemical Sciences, Dublin City University, Glasnevin, Dublin 9, Ireland
E-mail: silvia.giordani@dcu.ie
There are many issues associated with free drug delivery—the most prominent of which include: adverse side-effects, multi-drug resistance, premature drug degradation, lack of tissue penetration, and non-specific toxicity. Targeted delivery, which utilises nanocarriers as payload delivery vesicles, has the potential to address and alleviate these prominent issues. Specifically, it involves nanomaterials functionalised with targeting agents, allowing for the selective uptake of these nanocarriers by cells overexpressing specific receptors. This approach explicitly increases the drug concentration in the target cell of interest whilst minimising the exposure of healthy cells to the therapeutic agent. In this presentation, carbon nano-onions (CNOs) will be discussed as a potential vesicle for nanocarrier-type drug delivery systems.[1] CNOs, or multi-layer fullerenes, consist of multiple concentric shells of carbon atoms and are emerging as platforms for biomedical applications because of their ability to be internalized by cells and low toxicity both in vitro and in vivo. In my research group we have developed a synthetic multi-functionalisation strategy for the introduction of different functionalities (receptor targeting unit, imaging unit and drugs) onto the surface of the CNOs. The modified CNOs display high brightness and photostability in aqueous solutions and are selectively taken up by different cancer cell lines without significant cytotoxicity.

Silvia Giordani joined the School of Chemical Sciences at Dublin City University as Professor Chair of Nanomaterials in 2018 and took on the role of Head of School in 2020. Previously she received her PhD in Chemistry from the University of Miami, USA and carried out postdoctoral research at Trinity College Dublin (TCD) and at the University of Trieste, Italy. In 2007 she received the prestigious President of Ireland Young Researcher Award and was a Research Assistant Professor at TCD from 2007 to 2013. In 2013 she founded and directed the new “Nano Carbon Materials” research lab at the Istituto Italiano di Tecnologia (IIT) and in December 2016 she was appointed Associate Professor in Organic Chemistry at the University of Turin, Italy.
Her main research interests are in the design, synthesis, and characterization of a wide range of nanomaterials for applications in smart and responsive bio-related nanotechnologies. She is the author/co-author of more than 140 manuscripts, reviews and book chapters. She is the recipient of many international prizes and honours including the L’Oreal UNESCO for Women in Science fellowship, the William Evans visiting fellowship from the University of Otago (New Zealand) and is a Visiting Scientist to the Bio-Nano Institute at Toyo University (Japan). Her research has been recently featured in “Where I work” published in Nature on the 20th May 2021.
- 13:30 - 14:00 Lunch
- 14:30 - 15:45. Session V. Chair: Tamara Saksida
Analytical assays for assessment of NRF2 activation using HO-1 induction as a read-out for environmental stressors
Andreas Daiber
Department of Cardiology, Laboratory of Molecular Cardiology, University Medical Center of the Johannes Gutenberg University, Mainz, Germany
E-mail: daiber@uni-mainz.de
Environmental risk factors, including noise, air pollution, chemical agents, ultraviolet radiation (UVR) and mental stress have a considerable impact on human health. Oxidative stress and inflammation are key players in molecular pathomechanisms of environmental pollution and risk factors. Environmental pollutants are known to activate the nuclear factor erythroid 2-related factor 2 (NRF2), e.g. by induction of oxidative stress and inflammation. We here discuss the potential use of a heme oxygenase-1 promoter activity assay as a read-out of NRF2 activation by environmental toxins, e.g. reactive aldehydes in E-cigarette vapor or toxins such as heavy metals in particulate matter as well as the ultra-fine particles themselves. We also critically evaluate this heme oxygenase-1 promoter activity assay in comparison to other high-throughput assays.

Andreas Daiber studied Chemistry at the University of Konstanz (Diploma in 1997), holds a PhD in Biochemistry (graduated in 2000), did his habilitation (graduated in 2006), and since 2008 is a full professor in Molecular Cardiology at the University Medical Center Mainz. >33 significant research grants from the pharmaceutical industry and public funding bodies. 2011 guest professorship at the Université Joseph Fourier at Grenoble, France. From 2014-2016 Chair of COST Action BM1203 (EU-ROS). Memberships in national and international scientific communities (SFRBM/SFRRE, ASBMB, DGK), reviewer activities for numerous scientific journals (e.g. FRBM, Redox Biology, ATVB, Eur. Heart J., Nat. Comm.) and funding bodies, editorial board positions Cardiovasc. Res., Antioxidants, FRBM, Redox Biology), guest editor (Oxid. Med. Cell. Longev.,(Antioxid. Redox Signal., Redox Biology, Br. J. Pharmacol., FRBM, Antioxidants). He published >185 original research articles, >144 review articles, 25 book chapters, >160 conference abstracts and 3 patents with Boehringer Ingelheim. h-index: 71; >14,000 citations. Special research interests: redox biochemistry, oxidative stress and environmental research in cardiovascular disease.
Dissecting the role of oxidative stress on Nrf2 activity using biosensors and chemogenetic tools
Emrah Eroğlu
Research Institute for Health Sciences and Technologies (SABITA), Istanbul Medipol University, Türkiye
E-mail: emrah.eroglu@medipol.edu.tr
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a redox-sensitive transcription factor controlling antioxidant gene expression. Hydrogen peroxide H2O2 is a relatively stable and mild oxidant essential for signaling and metabolic pathways. Literature evidence establishes a critical role for H2O2 because it modulates the activity of Nrf2-dependent proteins (i.e., KEAP1). However, the exact role of H2O2 on NRF2 activation is controversial. The limited analytical tools available to visualize the multifaceted Nrf2 activation pathways undermine establishing the exact role of subcellular H2O2 levels. We pursue live-cell imaging approaches using genetically encoded biosensors to test the activation of Nrf2 downstream genes with the well-established pTRAF biosensors (plasmid for transcription factor reporter activation based upon fluorescence). Auranofin, a potent thioredoxin reductase inhibitor, is capable to induce H2O2 accumulation in cultured cells as documented by HyPer biosensor. Our results confirm that auranofin also causes robust activation of Nrf2 visualized with pTRAF biosensors. To link the role of H2O2 with the activation of Nrf2, we exploited chemogenetic tools to modulate the subcellular redox tone. Unexpectedly, neither exogenous administration of H2O2 nor endogenous generation of ROS levels with chemogenetic means did trigger Nrf2 activation despite eliciting strong levels of subcellular H2O2. Our findings are unexpected, shed light on the complexity of Nrf2 pathways, and draw a new picture of oxidative stress-regulated antioxidant response in cultured cells.

Emrah Eroğlu is an Assistant Prof. at Istanbul Medipol University and deputy director of the Research Institute for Health Sciences and Technologies (SABITA) in Türkiye. His laboratory develops genetically encoded biosensors and chemogenetic tools to visualize ROS and RNS- dependent pathways in vascular cells. He has published several original and review papers on biosensors, chemogenetic tools, and the implication of ROS pathways in RNS signaling, primarily in the context of vascular cells. His team is also developing novel animal model systems of vascular dysfunction linked to neurodegenerative diseases.
Effect of physiological cell culture media on cell viability and NRF2 activation
Anton Terasmaa
Laboratory of Chemical Biology, National Institute of Biophysics and Chemical Physics, Tallinn, Estonia
E-mail: anton.terasmaa@kbfi.ee
Cell models play a central role in preclinical research aimed at the mechanism of disease and drug discovery. Alterations in cell antioxidant response and bioenergetics profile are at the root of many diseases. Thus, the in vitro model of disease must also faithfully reproduce these disturbances. The activity of bioenergetic pathways and antioxidant response is regulated by the outside environment of the cells, including levels of nutrients and oxygen tension. At the same time, the composition of commonly used cell media deviates significantly from the composition of interstitial fluid in our body. Additionally, traditional cell culture work in done at ambient oxygen pressure (21%) while oxygen concentration in most tissues is just around 4-5%.
This study aimed to evaluate the effect of cell culture media and oxygen pressure on cell viability and antioxidant response. We changed the media of the cell cultures from DMEM to Plasmax, composition of which is similar to human plasma. Results show, that sensitivity of cells to various stressors (hydrogen peroxide, inducers of ferroptosis, inducers of ER stress) is different when cells are grown in physiologically relevant media as compared to conventional DMEM. For example, rat astrocytes (C6 cells) showed two orders of magnitude enhanced sensitivity to hydrogen peroxide when grown in Plasmax as compared to DMEM. Dose response of various stressors on activation of NRF2 was compared in HEPG2-ARE cells grown in Plasmax and DMEM in atmosphere of 5% and 19% oxygen. Result suggest that media composition has strong effect on cell antioxidant response.

Anton Terasmaa studied chemistry at the University of Tartu, Tartu, Estonia (1997) and neuroscience at Karolinska Institutet, Stockholm, Sweden (PhD 2004). Current position is senior research fellow at National Institute of Biophysics and Chemical Physics, Talllinn, Estonia Previous research interest was characterization of animal models of Wolfram syndrome and drug repurposing for this disease. Current research interest focuses on in-vitro modeling of neurodegeneration with brain iron accumulation (NBIA). Our aim is to develop a genetically modified cell model of a NBIA that can serve as a tool that will accelerate drug discovery for these disorders.
- 15:45 - 16:00. Coffee break
- 16:00 - 17:30. Poster session. Chair: Niki Chondrogianni
Neuropeptide Y promotes human M2 macrophage polarization through p62/SQSTM1- dependent autophagy and NRF2 activation
Elisabetta Profumo 1†, Elisa Maggi †2, Marzia Arese 3, Claudio Di Cristofano 2, Bruno Salvati 4, Luciano Saso 5, Rita Businaro 2 and Brigitta Buttari 1,*
1Department of Cardiovascular and Endocrine-Metabolic Diseases, and Aging, Istituto Superiore di Sanità, Rome, Italy 2Department of Medico-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Latina, Italy 3Department of Biochemical Sciences “A. Rossi Fanelli”, Sapienza University of Rome, Rome, Italy
4Department of Surgical Sciences, Sapienza University of Rome, Rome, Italy
5Department of Physiology and Pharmacology Vittorio Erspamer, Sapienza University of Rome, Rome, Italy
*brigitta.buttari@iss.it; +390649902635
† These authors contributed equally to this work
E-mail: brigitta.buttari@iss.it
Neuropeptide Y (NPY) is an abundantly expressed peptide capable of modulating innate and adaptive immune responses, regulating chemotaxis and cytokine secretion by macrophages. The inflammatory infiltrate within atherosclerotic plaque is characterized by accumulation of macrophages, which are subject to reprogram their phenotypes in response to environmental signals. Macrophage number and phenotype influence plaque fate. During atherosclerosis regression or stabilization macrophages switch from M1 pro-inflammatory phenotype to M2 anti-inflammatory reparative one. Here, we investigated whether NPY induces changes in phenotype and functions of human macrophages. Human monocytes were differentiated into macrophages with M-CSF (M0) and polarized towards M1 phenotype with IFN-γ plus LPS (M IFN) or M2 with IL-10 (M IL-10) and further challenged with NPY [10-7-10-9 M] for 8-36 hours. Cell phenotype and functions were analysed by molecular biology, immunofluorescence and immunochemical analyses. NPY affects macrophage surface markers and secretome profile expression thus shifting macrophages toward a M2-like phenotype. NPY also prevents the impairment of endocytosis, and prevents foam cell formation by reducing the lipid droplet accumulation in M0-polarized macrophages. NPY-treated M0 macrophages enhanced the autophagosome formation by upregulating the cell content of the autophagy marker LC3-II and p62-SQSTM1 and increased activation of the anti-oxidative transcription factor NRF2 (NF-E2-related factor 2), and the subsequent induction of its target gene HMOX1, that encodes heme oxygenase-1. Our findings indicate that NPY has a cytoprotective effect respect to the progression of the inflammatory pathway, both enhancing p62/SQSTM1-dependent autophagy and the NRF2-antioxidant sgnalling in macrophages.

Brigitta Buttari studied Biological Sciences at Sapienza University of Rome (in 1999), holds a postgraduate certification in Applied Biotechnology at Sapienza University of Rome (in 2003) and a PhD in Medical Microbiology and Immunology at Tor Vergata University of Rome (in 2008). Since 2008 is researcher at Istituto Superiore di Sanità (ISS- Italian National Institute of Health) in Rome, Italy. Her main research interest is focused on basic and translational research in the field of age-related diseases as atherosclerosis, a chronic and inflammatory condition characterized by oxidative stress and inflammation. A special research interest refers to study how certain oxidative modifications on endogenous molecules may affect the biology of human immune cells. She is seeking for endogenous and natural compounds that may act as oxidative stress and autophagy/apoptosis regulators through modulation of the Keap1-NRF2-ARE signalling pathway and thus may use to modulate the M1/ M2 macrophage balance on human and animal immune cell models.
Beneficial role of moderate ROS increase: an improved liver cell survival through stress response PACOS
Irina Milisav1,2,*, Izak Patrik Miller1, Iza Oblak1, Dušan Šuput1
1Institute of Pathophysiology, Faculty of Medicine, University of Ljubljana, Slovenia 2Faculty of Health Sciences, University of Ljubljana, Slovenia
E-mail: irina.milisav@mf.uni-lj.si
H2O2 is the main redox signalling and redox regulation molecule in the body. Its increase as a response to moderate stress can trigger a stress response in primary liver cells (hepatocytes). This prevents the triggering of apoptosis through caspase-9, which is the main apoptosis inducer in hepatocytes even for the external apoptotic signals. This beneficial stress response is called preapoptotic cell stress response (PACOS).
PACOS stress response that is manifested by an increased amount of ROS production and lower apoptosis triggering is reversible in primary hepatocytes, also by antioxidants, like N-acetylcysteine (NAC). The liver cells’ function is preserved at all times, in both, stress-adapted and normal cells. Therefore, the moderate increase of H2O2 can induce the reversible stress response that prevents the cells from unnecessary apoptosis.

Irina Milisav is a full professor of Biochemistry and Molecular Biology at University of Ljubljana, Slovenia, employed at Faculty of Medicine, Institute of Pathophysiology as a Research Associate and at Faculty of Health Sciences on a teaching post. She studies adaptive stress responses that boost cellular defences in liver cells (hepatocytes). Her group discovered an adaptive stress response of hepatocytes that prevents unnecessary apoptosis triggering when the stress-adapted hepatocytes are exposed to subsequent moderate stress (PACOS). ROS signalling mobilizes antioxidative defence and PACOS. Her group also investigates the effects of selected second-generation antipsychotics on the liver as a part of a Marie Sklodowska Currie ITN project and the role of stress responses in drug-induced liver injury (DILI). She is a Grant Awarding Coordinator at COST CA20121 BenBedPhar Action.
Long-term exposure to hydrogen peroxide modulates NRF2 and AQP3 in breast cancer cell lines
Monika Mlinarić, Ivan Lučić, Lidija Milković, Ana Čipak Gašparović
Institute Ruđer Bošković, Croatia
E-mail: Monika.Mlinaric@irb.hr
H2O2 is the main redox signalling and redox regulation molecule in the body. Its increase as a response to moderate stress can trigger a stress response in primary liver cells (hepatocytes). This prevents the triggering of apoptosis through caspase-9, which is the main apoptosis inducer in hepatocytes even for the external apoptotic signals. This beneficial stress response is called preapoptotic cell stress response (PACOS).
PACOS stress response that is manifested by an increased amount of ROS production and lower apoptosis triggering is reversible in primary hepatocytes, also by antioxidants, like N-acetylcysteine (NAC). The liver cells’ function is preserved at all times, in both, stress-adapted and normal cells. Therefore, the moderate increase of H2O2 can induce the reversible stress response that prevents the cells from unnecessary apoptosis.

MonikaMlinarić is a PhD student working in the Laboratory for Oxidative Stress, Division of Molecular Medicine at Ruđer Bošković Institute. She is working on the project “Elucidating the role of aquaporins 3 and 5 in the development of breast cancer resistance to oxidative stress (AquaBCaRe)” with Dr. Lidija Milković and Dr. Ana Čipak Gašparović. As a topic of her PhD, she will investigate the interaction of Nrf2 and aquaporins (channels that facilitate the transport of hydrogen peroxide across the membrane), as well as their response to the state of chronic oxidative stress.
Role of transcription factor NRF2 in the regulation of the Blood-Brain Barrier component TIE2 receptor
Eduardo Cazalla
Instituto de Investigaciones Biomédicas “Alberto Sols” UAM-CSIC, Department of Biochemistry, School of Medicine, Autonomous University of Madrid, Spain.
E-mail: ecazalla@iib.uam.es
The Blood-Brain Barrier (BBB) participates in the selective permeability of molecules that are delivered from the bloodstream to the brain. One key player in maintaining the BBB properties is the ANGPT1/2-TIE2 signalling pathway. The breakdown of BBB integrity has long been linked to neuropathological situations, where endothelial cells are subjected to oxidative and inflammatory stress. NRF2 is known to activate a genetic program for defence against multiple stress responses and could participate in the preservation of the BBB. However, the relationship between NRF2 and the ANGPT1-TIE2 pathway has not been hardly explored. We studied the effect of chemical and genetic activation of NRF2 on TEK/Tek expression, encoding TIE2, after an in-silico search resulting in 2 ARE regions located in the gene sequence. NRF2 activation by sulforaphane or by overexpression resulted in a decrease in TIE2 levels in endothelial cells derived from the neurovascular unit. By contrast, the transcription factor BACH1, a classical repressor of the NRF2 activity, presented the opposite effect promoting TEK/Tek regulation. The effect of the NRF2/TIE2 axis on endothelial cell functions was compared with a lipopolysaccharide (LPS) inflammation model, which also lead to a decrease in TIE2 levels. While most ARE-genes are activated by NRF2 and repressed by BACH1, our results suggest that TEK/Tek belongs to a reduced number of ARE-genes that exhibit the opposite regulation.

Eduardo Cazalla studied Biology at the Autonomous University of Madrid (2021) and is starting his PhD studies under the supervision of Dr. Antonio Cuadrado and Dr. Ángel Juan García-Yagüe, working on the project “The transcription factor NRF2 in Alzheimer’s disease pathophysiology”. For his PhD he will continue the research started in his master’s thesis studying the relationship between NRF2 and BBB stability mediated by the ANGPT1-TIE2 pathway in an Alzheimer’s disease context.
Regulation of the extracellular matrix glycoprotein Reelin by the transcription factor NRF2
Ángel Juan García-Yagüe
Instituto de Investigaciones Biomédicas “Alberto Sols” UAM-CSIC, Departament of Biochemistry, School of Medicine, Autonomus University of Madrid, Spain.
Email: ajgarcia@iib.uam.es
Dysregulation of oxidative metabolism is associated with structural and functional changes in the extracellular matrix (ECM) that have been associated with the pathogenesis and progression of several neuropsychiatric and neurodegenerative disorders. A crucial protein of the ECM is Reelin, a large glycoprotein that plays essential roles in the developing and adult brain. Reelin is cleaved at two well-defined sites that generate N-terminal (N-R2), central, and C-terminal fragments. The selective cleave by well-established specific proteases provides several signalling mechanisms in the brain parenchyma that are disrupted by redox alterations.
Transcription factor NRF2, nowadays considered the master regulator of the antioxidant response, activates a battery of antioxidant and cytoprotective genes, and, therefore, it should impact the ECM. Here, we studied if NRF2 might modify the maturation of Reelin by targeting the expression of genes encoding ECM proteases. We found that in cultured neurons and astrocytes the NRF2 activator sulforaphane reduces the cleavage of Reelin that generates the N-R2 fragment. This reduction correlates with a decrease in the transcript and protein levels of several ECM proteases. These effects were further studied in prenatal brains and cortex-hippocampus of adult NRF2- knockout mice, where we found an increase in the levels of the N-R2 fragment and in several metalloproteinases, that are not observed in wild-type mice.
The control of Reelin cleavage by NRF2 provides a new and very relevant layer of regulation for brain homeostasis.

Ángel Juan García-Yagüestudied Biochemistry at the Autonomous University of Madrid (2006), holds a PhD in Biochemistry (2012), and since 2012 had been hired as a scientific researcher in the same university under the supervisor of the Cuadrado lab. During his professional career, he had focused at to study and understanding the neurological process that drives neurodegenerative pathology disease, on focus Parkinson’s disease, through the physiopathological mechanics study of the transcription factor NURR1. Currently, he is leading several research projects to focus on the NRF2 role in brain physiology and pathology related to blood-brain-barrier integrity and extracellular matrix remodelling. Besides, he is implicated in research about novel pharmacological approaches in preclinical models of neurodegenerative diseases.
Nuclear Factor Kappa B (NF-𝜅B), a redox-sensitive transcription factor and Fibroblast Growth Factor 21 (FGF21), an NRF2-related regulator of oxidative stress cell responses, in health-to-disease transition in non-communicable diseases
Christina Morgenstern1, Gernot Faustmann1,2, Beate Tiran3, Johannes M. Roob2 and Brigitte M. Winklhofer-Roob1
1 Institute of Molecular Biosciences, University of Graz, Austria; 2 Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Austria; 3 Clinical Division of Nephrology, Department of Internal Medicine, Medical University of Graz, Austria
E-mail: christina.morgenstern@uni-graz.at
Background: NF-𝜅B and NRF2 are the two major transcription factors that regulate cellular responses to inflammation and oxidative stress, respectively. Both pathways have been shown to be functionally connected, thereby mediating fine-tuning of dynamic changes under both physiological and disease conditions. The stress-responsive hormone FGF21 is related to NRF2 and NF-𝜅B and exerts protective effects by reducing reactive oxygen species (ROS) formation and inflammation. It plays a key role in glucose and lipid metabolism as well as in the control of energy balance.
Study aims: Based on the hypothesis that circulating levels of FGF21 are affected already in early stages of metabolic disturbances in non-communicable diseases in comparison to full health and irrespectively of age, we investigated a possible role of FGF21 in health-to-disease transition, using metabolic and oxidative stress biomarkers in plasma, red blood cells (RBC) and peripheral blood mononuclear cells (PBMC). A panel of biomarkers was selected to cover possible involvement of FGF21 in different organs.
Subjects: Apparently healthy subjects of the BIOCLAIMS cohort with mildly impaired renal, vascular, or metabolic health were studied and compared to subjects with clinically and biochemically proven full health. Overlaps between groups were excluded to allow for identifying FGF21 behaviour in the four different groups.
Results: Our results show that subjects with impaired renal function and metabolic health had higher FGF21 plasma levels compared to subjects with impaired vascular function and full health. In addition, subjects with impaired renal function and metabolic health also showed higher levels of branched-chain amino acid-derived C5-acylcarnitine (suggesting incomplete fatty acid oxidation), leptin, retinol-binding protein 4, triglycerides and fatty liver index, BMI, and waist circumference. This contrasted with lower HDL cholesterol, ascorbate and β-cryptoxanthin in these groups. In addition, NF-𝜅B activation, both p50 and p65, and human mercaptalbumin (reduced form) were lower in subjects with impaired renal function compared to all other groups.
Conclusions: These results demonstrate that circulating FGF21 levels are elevated in early stages of metabolic disturbances including adiposity, insulin resistance and impaired kidney function, but not in early vascular changes in the absence of such disturbances, underpinning FGF21 as a highly relevant metabolic node.

Christina Morgenstern studied Molecular Microbiology at the University of Graz (Diploma in 2004) and holds a PhD in Developmental Genetics from University College London (graduated in 2009). Pursued PhD work at the Cancer Research UK London Research Institute (now: The Francis Crick Institute) under the supervision of Dr. David Ish-Horowicz FRS and studying the transcriptional regulation of Notch signalling in vertebrate embryogenesis. In addition to biomedical background, she holds an MSc in Data Science with focus on Computational Biology (graduated in 2021). Currently a Postdoctoral fellow at the University of Graz and a lecturer for biology at the University College of Teacher Education Carinthia and the University of Graz. From 2022 Chair of COST Action
BenBedPhar working group 3, Translational Medicine. Memberships in international scientific communities (Society for Free Radical Research Europe, International Society for Computational Biology, Human Cell Atlas Consortium) Special research interests: cell signalling, transcriptional regulation, analysis of omics data
Assessment of the anti-inflammatory effects of extracellular vesicles in human microglial cells
Virginijus Tunaitis, Diana Romenskaja, Justina Pajarskienė J, Barbora Lekešytė, Augustas Pivoriūnas
Department of Stem Cell Biology, State Research Institute Centre for Innovative Medicine, LT-01102, Vilnius, Lithuania.
Email: virginijus.tunaitis@imcentras.lt
Extracellular vesicles (EVs) represent a new therapeutic approach to control and cure a number of neurodegenerative diseases. Our previous data demonstrated therapeutically beneficial role of EVs in rat model of Parkinson’s disease (PD). In this in vivo model intranasal administration of EVs prevented 6-hydroxydopamine (6-OHDA) induced loses of dopaminergic neurons in substantia nigra region of rats brain. However possible EV targets in the brain and their therapeutically beneficial response mechanism remains to be elucidated.
In this study we analysed influence of EVs on microglial cells – an important regulator of inflammatory processes in the brain. For this we used EVs, derived from human exfoliated deciduous teeth stem cells (SHEDs), and immortalized human microglial cell line as a target cells. Analysis of expression pattern of cytokines, transcription changes of inflammation related genes revealed anti- inflammatory effects of EVs. Finally, to quantify the influence of EVs on inflammatory processes in the cell, we established NFκB and NRF2 reporter plasmids. Overall, our data confirm an anti- inflammatory effect of EVs and enable us to assess EVs suitability for future therapeutic usage.

Virginijus Tunaitis is senior researcher at the Department of Stem Cell Biology, Centre for Innovative Medicine, Vilnius. He is involved in research related to the immunomodulatory properties of mesenchymal stem/stromal cells (MSCs) and the importance of paracrine mechanisms in therapeutic action of MSCs.
The expression pattern of inflammation and redox genes in the blood of patients with cardiovascular pathology
Elena Milanesi1*, Gina Manda1, Maria Dobre1, Ionela Victoria Neagoe1, Antonio Cuadrado2
1Radiobiology Laboratory, “Victor Babes” National Institute of Pathology, Bucharest, Romania 2Department of Biochemistry, Faculty of Medicine, Autonomous University of Madrid, Madrid, 28049, Spain
E-mail: elena.k.milanesi@gmail.com; elena.milanesi@ivb.ro
Extensive evidence indicates a critical role of low-grade chronic inflammation and oxidative stress as active participants in the pathological mechanisms underlying non-communicable diseases (NCDs). Inflammation and redox biomarkers might therefore be useful in disease prognosis and therapy response. This study aimed to identify gene expression changes in blood that might underlie pathologic processes in elderly patients with various NCDs (cardiovascular disease, hypertension, dyslipidemia including hypercholesterolemia, type 2 diabetes mellitus), kept under control by polyvalent disease-specific medication. We analyzed the expression of 168 inflammation- and redox- related genes in the blood of a cohort of 130 elderly patients. The study identified a down-regulation of the inflammation-related genes NFKB2, NFKBIA, RELA, RELB, AKT1, IRF1, STAT1, CD40, LTA, TRAF2, PTGS1, ALOX12, and the following redox –related genes, DUOX1, DUOX2, MPO, GSR, TXNRD2, HSPA1A, MSRA and PDLIM, in patients with cardiovascular disease (CVD). The study strongly suggests that this specific panel of under expressed inflammation- and redox- related genes can be used for non-invasively monitoring disease progression and patient engagement to medication in CVD.

Elena Milanesi is a researcher at the National Institute of Pathology Victor Babes, Bucharest, Romania. She studied Medical Biotechnologies at the University of Brescia Italy, where she holds a PhD in Molecular Genetics Applied to Medical Sciences (2012). She worked two years at the Sackler Faculty of Medicine of Tel Aviv, Israel as Post-Doc. She is partner coordinator of the project “Burning mouth syndrome-an interdisciplinary approach for diagnosis and disease monitoring” (2021-2023) and she is Principal Investigator of the ICRP/ICGEB project “The brain-gut axis linking inflammatory bowel disease with anxiety and depression: the inflammation-microbiome network” (2022-2024). Since October 2021 she is Gender Equality Officer of the COST ACTION CA2012.
- 16:30 - 18:30. Networking for joint projects
- 18:30 - 18:45. Concluding remarks. Chair: Prof. Antonio Cuadrado, Spain
- 20:30. Gala dinner